|
|
Journal of Advanced Veterinary Research Volume 9, Issue 1, 2019, Pages 23-28 |
|
|
Estimation of Bone Marrow DNA Damage Induced by Lambda cyhalothrin and Dimethoate Insecticides using Alkaline Comet Assay |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Doha Yahia1, Marwa F. Ali2, Doaa S. Abd El-Maguid3 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Assiut University, Egypt 2Department of Veterinary Pathology and Clinical Pathology, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 3Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, New Valley University, Egypt (Corresponding author; safwat_doaa79@yahoo.com). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Received: 22 November 2018, Accepted: 27 December 2018 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Dimethoate (DM) and Lambda cyhalothrin (LCT) are commonly used insecticides. Human being and farm animals are expected to have acute toxicity. The present work aimed to explore the effect of acute exposure to DM and LCT on hematological parameters and to detect DNA damage in bone marrow of Sprague Dawley rats using the alkaline single cell gel electrophoresis assay (comet assay). Thirty animals were divided into three groups of ten rats each. LCT group administered 26 mg/kg body weight, DM group administered 103 mg/kg body weight orally for 24 and 48 hours, while the control group received the vehicle only. Blood samples were collected for hematological analysis, bone marrow was flushed from the femur bone for comet assay and spleen samples were preserved in formalin for histopathological examination. Results showed minor changes in blood profile in all exposed groups associated with mild changes in histology of spleen tissue. Alkaline single cell gel electrophoresis assay in bone marrow cells showed that LCT and DM caused extensive and severe DNA damage after 48 h exposure expressed as significant increases in all comet parameters (% DNA in tail, tail length, tail moment and Olive tail moment). The results concluded that LCT and DM induced DNA damage in bone marrow of rats, LCT showed higher degree of DNA damage in comparison with DM. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Keywords: Lambda cyhalothrin; Dimethoate; DNA damage; Bone marrow; Spleen; Blood |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Introduction |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Pesticides are extensively used in agricultural fields to enhance food production through eradication of unwanted insects and controlling disease vectors (Anwar, 1997). Among the most common insecticides, synthetic pyrethroids (lambda cyhalothrin) and organophosphate compounds (dimethoate) are widely used in agriculture and industry all over the world (Sharma et al., 2005). Lambda cyhalothrin (LCT) is a synthetic pyrethroid insecticide with effective activity against a large variety of arthropods, which are harmful to human and animal health. LCT is known as a potent neurotoxicant, characterized by high insecticidal properties and low mammalian toxicity (He et al., 2008). Exposure to LCT can induce both acute and chronic risks; acute effects include eye and skin irritation, non-cardiogenic pulmonary edema, cardiovascular toxicity, convulsions, coma and severe muscle fasciculation (Iqbal et al., 2007). Chronic effects include decreased body weights, organ weight changes (liver, kidney, brain, heart and lung), reduced brain size (Kohner et al. 1999) cell damage (neoplastic and histopathological lesions), tumors, endocrine toxicity (Kothari et al., 2002) and DNA damage in liver and brain (Yahia and Ali, 2018). Dimethoate (DM) behaves like any other organophosphate compound as it has anticholinesterase activity (Costa, 2006). Inhibition of brain acetyl cholinesterase results in accumulation of acetylcholine and hyper activation of acetylcholine receptor at neuromuscular junction and in the autonomic and central nervous system. This will be manifested as convulsions and tremors leading to death in severe cases (Lotti, 2001). Dimethoate exerts several toxic effects on different tissues and organs including liver, brain (Sharma et al., 2005; Sayim, 2007; Astiz et al., 2009), pancreas (Hagar and Fahmy, 2002) and kidney (Mahjoubi et al., 2008). Dimethoate was reported to cause malignant and benign neoplasms in the liver, lymphatic system and endocrine organs and induced DNA damage in human lymphocytes (Undeger and Basaran, 2005). Furthermore, acute exposure to DM caused DNA damage in brain and liver of rats (Yahia and Ali, 2018). Comet assay (single cell gel electrophoresis) is a simple, rapid and sensitive procedure for measuring DNA damage in mammalian cells and has been widely used for genotoxic studies. (Singh et al., 1988; Tsuda et al., 1998). The most commonly used parameters of comet features are the tail parameters such as tail length, tail moment and the % tail DNA (De Boeck et al., 2000). Among these parameters, tail DNA was a more appropriate parameter than tail length to analyze induced DNA damage (De Boeck et al., 2000). These parameters were used for genotoxic studies by many researchers (Sasaki et al., 1997; Kim et al., 2002; Anderson et al., 2003; Schabath et al., 2003). Toxicity of human and animals from insecticides usually occurs in an acute form due to exposure to a single large dose; recent exposure to xenobiotics may be reflected in change in blood profile. Bone marrow is the factory of blood cells production and has a large number of dividing cells; it is a suitable tissue for detection of DNA damage by using comet assay. This study aimed to investigate the acute effect of lambda cyhalothrin and dimethoate insecticides on hematological parameters, DNA damage in bone marrow and histological changes in spleen. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Materials and methods |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Chemicals Lambda cyhalothrin 2.5 % (Dolf 2.5 EC) a synthetic pyrethroid insecticide (Star Chem. Co., Egypt) and Dimethoate 40% (Saydon/ Cheminova 40% EC) organophosphate insecticides (Kafre Elzayat, KZ Co., Egypt) were used in the study. The commercial formulations were used in the current study because they contain organic solvents, surface active ingredients and activity enhancers with poorly characterized toxicity. These products are often highly toxic than the technical grades of pesticide compound (Ducolomb et al., 2009). Animals and treatment A total number of 30 healthy adult Sprague Dawley male rats with body weight ranged from 100 - 130 g, were used in this study. Rats were obtained from the Experimental Animal Center, Faculty of Medicine, Assiut University, Egypt. Rats were divided into three groups of ten animals each, housed in plastic cages and allowed to acclimatize for a week before treatment. Animal facilities were controlled for temperature (24–26°C) and operated under a 12 hours light-dark cycle. Rats were fed on standard food pellets and tap water ad libitum. The insecticides LCT and DM were dissolved in corn oil. The first group was exposed to 26 mg/kg body weight LCT [oral LD50 79 mg/kg body weight (US Environmental Protection Agency, 1988)]. The second group was exposed to 103 mg/kg body weight DM [oral LD50 310 mg/kg (JMPR, 1998)]. The third group was left as control and received only corn oil. All treated animals were exposed to 1/3 LD50 orally for 24 and 48 hours. All applicable national and institutional guidelines for the care and use of animals were followed. Handling, use, and care of experimental animals were done according to the ethical guidelines for animal experiments in Assiut University, Egypt. Necropsy and sampling Five animals from each group were euthanized under diethyl ether anesthesia 24 h after the first exposure, whereas another five animals were euthanized 24 h after administration of the second dose (48 h). Blood samples were collected from the descending aorta in vacutainer tubes coated with EDTA anticoagulant for hematological analysis. Bone marrow was flushed out from each femur bone using 1 ml of homogenizing buffer (0.075 M NaCl and 0.024 M Na2EDTA), centrifuged at 1500 rpm for 10 min. at 0°C, the supernatant was re-suspended and then used for comet assay. Spleen samples were collected and kept in 10% neutral buffered formalin for histopathological examination. Hematological analysis Hematological examination of blood samples was performed by using Medonic Vet. Hematology analyzer (Medonic CA 620, Sweden). Single cell gel electrophoresis (SCGE), comet assay Comet assay was used to detect DNA damage in bone marrow according to Sasaki et al. (1997) as follows: Slide preparation Frosted slides were layered twice with 100 µL 1% GP-42 normal agarose (Nacalai Tesqe, Inc. Kyoto, Japan). Seventy-five microlitre (µL) of nuclear suspension was mixed with equal amount of 2% low melting (LGT) agarose (Nacalai Tesqe, Inc. Kyoto, Japan) at 45°C and the mixture was layered on the frosted slide using a cover slide. Then, 100 µl of agarose GP-42 was layered on the surface and covered with another slide and allowed to gel. Lysing The slides were placed immediately into a chilled lysing solution (2.5 M NaCl, 100 mM Na4EDTA, 10 mM Trizma, 0.1% Sodium lauryl sulfate (SDS), 10% dimethyl sulfoxide, and triton X-100) and kept at 4°C in the dark for 60 minutes. Unwinding and electrophoresis The slides were arranged on a horizontal gel electrophoresis platform (Cleaver Scientific Ltd, U.K.) and covered with chilled alkaline solution (300 mM NaOH and 1 mM Na2 EDTA, pH 13) in the dark for 10 min, electrophoresis was conducted at 25V and approximately 300 mA for 15 min. then the slides rinsed with 400 mM Tris buffer (Wako Pure Chemical Industries, Ltd. Japan) pH 7.5 for 7 min. to neutralize the excess alkali. The neutralized slides were kept in ethanol for 5 min. then allowed to dry at room temperature, then stained with 50 µL (20 µg/ml) ethidium bromide (Wako Pure Chemical Industries, Ltd.). Examination of the Slides The nuclei on the slides were examined at 200 fold magnification using the green filter of a fluorescence microscope (Olympus BX-43, Japan). The image of the nuclei was captured using digital camera. At least 50 cells per slide were analyzed using Comet Assay Software Project (CASP) to measure the diameter of the head, % of DNA in the tail and the length of tail of the comet to obtain DNA migration. Tail moment (TM) and Olive tail moment (OTM) were used as accurate indicators of DNA damage; Tail moment = Tail length X %Tail DNA/100 Olive Tail Moment = (Tail mean – Head mean) X %Tail DNA/100 Histopathological examination Fresh specimens from spleen of rats from all experimental groups were collected and fixed in 10% neutral buffered formalin. The tissues were dehydrated in a graded alcohol series, cleared with methyl benzoate, embedded in paraffin wax, sectioned at 4 µ thickness and stained with hematoxylin and eosin, histopathological examination by light microscopy (Olympus CX31, Japan) and photographed using digital camera (Olympus, Camedia C-5060, Japan) (Bancroft et al., 1996). Statistical analysis Statistical analyses were done using SPSS software package version 16.0. Data were analyzed by using one-way analysis of variance (ANOVA) followed by post-hoc lowest significant difference (LSD) multiple range test for comparison between control and exposed groups. All data were represented as mean±SD for all experimental and control animals (P < 0.05). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Results |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Hematological analysis Exposure to LCT for 24 h showed significant increases (P< 0.01) in hematocrit (HCT %) and (P< 0.05) mean corpuscular volume (MCV), while other parameters showed no significant changes. DM exposure did not show any change except mild increase in mean corpuscular hemoglobin concentration (MCHC) at 24 h., on the other hand, white blood cells (WBCs), lymphocytes, granulocytes and monocytes were increased after exposure to DM for 48 h (Table 1). Thrombocytic parameters (Table 1) were normal in all exposed groups, except group that exposed to DM for 24 h, which showed significant (P<0.05) increases in mean platelet volume (MPV) and in platelet distribution width (PDW). Table 1. Effect of exposure to lambda cyhalothrin and dimethoate for 24 and 48 hours on the hematological parameters of male Sprague Dawley rats.
Data are shown as mean ± SD. N= 5 rats/ group. Under each treatment: values followed by different superscript means significant compared to the control group. (P<0.05). Letters (a and b) were used to compare LCT group with control group, letters (c and d) were used to compare DM group with the control group. DNA damage in bone marrow Exposure to LCT for 24 h did not show any significant changes in bone marrow DNA in comparison with the control group, while exposure for 48 h induced severe DNA damage indicated by significant increases (P<0.01) in tail moment, olive tail moment, tail length and % of migrated DNA in tail (Fig. 1A and B and Table 2). On the other hand, exposure to DM for 24 h showed significant (P< 0.05) increase in tail length only, while 48h exposure caused extensive DNA damage and significant increases (P< 0.01) in tail moment, olive tail moment, tail length and % of DNA in tail (Fig. 1C and D and Table 2). Table 2. DNA damage in bone marrow of rats exposed to lambda cyhalothrin for 24 h and 48h.
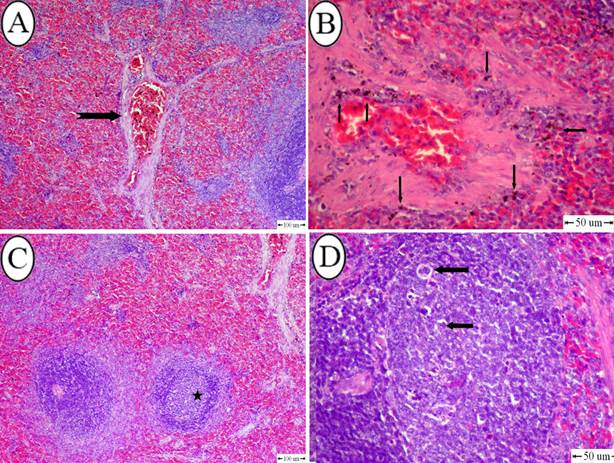
Data are shown as mean ± SD. N= 5 rats/ group. Under each treatment: values followed by different superscript means significant compared to the control group. (P<0.05). Letters (a and b) were used to compare LCT group with control group, letters (c and d) were used to compare DM group with the control group. Fig. 1. A) Bone marrow nuclei of rats exposed to 26 mg/kg body weight of LCT for 24 h showing mild DNA damage. B) LCT group exposed to 26 mg/kg body weight of LCT for 48 h showing severe DNA damage. C) Bone marrow nuclei of rats exposed to 103 mg/kg body weight of DM for 24 h showing mild DNA damage, while exposure for 48 h induced severe DNA damage (D). Histopathology of spleen Mild histopathological changes were observed in all the exposed groups; exposure to LCT for 24 h resulted in slight congestion in some blood vessels, with no changes in organization of white and red pulps (Fig. 2A). However, after 48 h exposure, the vascular changes became more evident in the form of congestion in most of blood vessels associated with marked hemosiderosis (Fig. 2B). In DM group, few follicles showed mild depletion after 24 h exposure (Fig. 2C). On the other hand, the depletion in follicles were increased after exposure to DM for 48 h, which appeared as vacuolation in lymphocytes with disorganization in white pulp (Fig. 2D).
Fig. 2. A) Spleen of rats exposed to lambda cyhalothrin (26 mg/kg body weight) for 24 h showing congestion of blood vessels (notched arrow) (H&E, bar= 100 um). B) Spleen, lambda cyhalothrin intoxicated group after 48h showing hemosidrosis (notched arrow) (H&E, bar= 50 um). C) Spleen of rats exposed to dimethoate (103 mg/kg body weight) for 24 h showing depletion in white pulp (star) (H&E, bar= 100 um). D) Spleen, dimethoate intoxicated group after 48h showing vacuolation in lymphocytes (star) (H&E, bar= 100 um). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Discussion Pesticides related poisonings has increased over the past years due to their uncontrolled use (Abdollahi et al., 2004). Synthetic pyrethroid and organophosphate insecticides are among the most commonly used chemicals for controlling pests. The current work revealed that exposure to DM for 24 and 48 h had no significant changes in the hematological parameters of rats except mild changes in leucocytes profile and thrombocytes at 48 h. This result is in accordance with Dogan and Can (2011), who found that exposure of fish to dimethoate for 5 days did not change the blood profile but longer exposure time showed significant changes in all blood parameters. Parkash (2016) reported that 96 h exposure to dimethoate could produce severe changes in blood profile. Histology of spleen showed mild changes, which appeared as depletion in white pulp at 24 h and vacuolation of lymphocytes at 48 h, these results may support the blood profile data. LCT exposure showed no change in blood picture while the spleen showed congestion of blood vessels at 24 h and hemosidrosis at 48 h. Abd Elkawy et al. (2013) found that exposure of rats to LCT for 45 days caused significant decreases in RBCs count, Hb and HCT concentration. The obtained results of hematology and histopathology of spleen for DM and LCT may be related to the short time of exposure (24 and 48 h), which was not enough to induce observable changes in hematological parameters and in the histology of spleen. In the present study, the genotoxic effect of lambda cyhalothrin and dimethoate in bone marrow cells was estimated by using comet assay, which is increasingly used in genotoxicity testing in vivo (Hartmann et al., 2001; Kiskinis et al., 2002) and in vitro (Ventura et al., 2008; Nwani et al., 2010) because it is rapid, simple, easy and highly sensitive assay (Tice et al., 2000). It has an advantage over other traditional cytogenetic assays by not needing mitotically active cell (Dogan et al., 2011). The current results showed that LCT and DM exposure for 24 h could not induce DNA damage in bone marrow cells. While 48 h exposure resulted in significant and marked increase in all DNA damage parameters (tail parameters), which include tail length, %DNA in tail, tail moment and olive tail moment. The percentage of DNA in the tail is strongly selected as the parameter of choice and directly related to DNA break frequency because it reflects the amount of migrated DNA from the nucleus (Dogan et al., 2011). On the other hand, tail moment calculation includes the percentage of migrated DNA to the tail multiplied by the tail length (Collins et al., 1997; Tice et al., 2000) so it is more accurate and indicates the intensity of DNA damage (Knopper et al., 2005). De Boeck et al., (2000) stated that the tail parameters that are the most frequently used are those of the tail length, tail DNA% and tail moment. Olive tail moment results were correlated with other parameters and it is also considered as a good indicator for DNA damage; it is calculated by the difference between mean tail and head multiplied by % tail DNA (Olive et al., 1990; Kumaravel and Jha, 2006). Boussema et al. (2012) reported that dimthoate caused DNA damage in bone marrow of mice in a dose dependent manner. Also, our previous study (Yahia and Ali, 2018) revealed that DM and LCT induced DNA damage in liver and brain of Sprague Dawley rats. Braun et al. (1982) stated that OP compounds have alkylating properties either direct alkylation or indirect via protein alkylation, which can induce DNA damage. On the other hand, WHO (1990) stated that the induced DNA damage by LCT might be due to the release of unstable cyanohydrins, which decompose to cyanides and aldehydes, and in turn could act as a source of free radicals that attack DNA and cause different degrees of DNA damage. The obtained results revealed that acute exposure of rats to LCT and DM for 48 h induced severe DNA damage in bone marrow cells. LCT showed higher degree of DNA damage indicated by longer tail length, higher tail moment and % DNA in tail. These data may be related to LD50 of each compound as LCT has lower LD50 (79 mg/kg) compared with DM (310 mg/kg). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conclusions The studied insecticides LCT and DM induce DNA damage in bone marrow of rats at 48 h, which accompanies with mild histopathological changes in spleen tissue. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conflict of Interests |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The author(s) declared no conflicts of interest in regarding to the research, authorship, and/or publication of this article. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
References |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abd Elkawy, M., Soliman, G.A., Abd-El Rehim, E., 2013. Effect of Solanum nigrum Linn against Lambda Cyhalothrin-Induced Toxicity in Rats. IOSR-JPBS 5, 55-62. Abdollahi, M., Mostafalou, S., Mohammadi, P., Shadnia, S., 2004. Oxidative stress and cholinesterase inhibition in saliva and plasma of rats following subchronic exposure to malathion. Comp. Biochem. Physiol. C 137, 29–34. Anderson, M., Agurell, E., Vaghef, H., Bolcsfoldi, G., Hellman, B., 2003. Extended-term cultures of human T-lymphocytes and the Comet assay: a useful combination when testing for genotoxicity in vitro?. Mutat. Res. 540, 43–55. Anwar, W.A., 1997. Biomarkers and human exposure to pesticides. Environ. Health Perspect 4, 801–806. Astiz, M., de Alaniz, M.J., Marra, C.A., 2009. Effect of pesticides on cell survival in liver and brain rat tissues. Ecotoxicol. Environ. Saf. 72, 2025-2032. Bancroft, D., Stevens, A., Turmer, R., 1996. Theory and practice of histological technique, 4th ed., Churchill Living Stone, Edinburgh, London, Melbourne. pp. 47-67. Boussema, A., Rjiba, K., Mnasri, N., Moussa, A., Bacha, H., 2012. Genotoxicity evaluation of dimethoate to experimental mice by micronucleus, chromosome aberration tests, and comet assay. Int. J. Toxicol. 31, 78-85. Braun R., Schoneich J., Weissflog L., Debek W., 1982. Activity of organophosphorus insecticides in bacterial tests for mutagenicity and DNA repair-direct alkylation vs metabolic activation and breakdown.I: butanoate, vinylbutonate, dichlorvos, demethyldichlorovos and demethyl vinylbutonate. Chem Biol Interact. 39, 339–350. Collins, A.R., Dobson V.L., Dusinská M., Kennedy G., Stĕtina R., 1997. The comet assay: what can it really tell us? Mutat. Res. 375, 183-193. Costa, L.G., 2006. Current issues in organophosphate toxicology. Clin. Chim. Acta 366, 1-13. De Boeck, M., Touil, N., De Visscher, G., Vande, P.A., Kirsch-Volders, M., 2000. Validation and implementation of an internal standard in comet assay. Mutat. Res. 469, 181–197. Dogan, D., Can, C., 2011. Hematological, biochemical, and behavioral responses of Oncorhynchus mykiss to dimethoate. Fish Physiol. Biochem. 37, 951–958. Dogan, D., Can, C., Kocyigit, A., Dikilitas, M., Taskin, A., Bilinc, H., 2011. Dimethoate-induced oxidative stress and DNA damage in Oncorhynchus mykiss. Chemosphere 84, 39–46. Ducolomb, Y., Casas, E., Valdez, A., Gonzalez, G., Altamirano- Lozano, M., Betancourt, M., 2009. In vitro effect of malathion and diazinon on oocytes fertilization and embryo development in porcine. Cell Biol. Toxicol. 25, 623-633. Hagar, H.H., Fahmy, A.H., 2002. A biochemical, histochemical, and ultrastructural evaluation of the effect of dimethoate intoxication on rat pancreas. Toxicol. Lett. 133, 161-170. Hartmann, A., Kiskinis, E., Fjaellman, A., Suter, W., 2001. Influence of cytotoxicity and compound precipitation on test results in the alkaline comet assay. Mutat. Res. 497, 199-212. He, L., Troiano, J., Wang, A., Goh, K., 2008. Environmental chemistry, ecotoxicity, and fate of lambda cyhalothrin, Rev. Environ. Contam. Toxicol. 195, 71-91. Iqbal, M.J., Bollaert, M., Chickris, N., James, B., Higginbotham, D.A., Peterson, R., Murphy, L., 2007. Ginseng modifies the diabetic phenotype and genes associated with diabetes in the male ZDF rat. Phytomedicine 14, 681–689. JMPR, 1998. Pesticide residues in food – 1998 Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, FAO Plant Production and Protection Paper 148, Rome. Kim B.S., Park J.J., Edler L., Von Fournier D., Haase W., Sautter-Bihl M.L., Gotzes F., 2002. New measure of DNA repair in the single-cell gel electrophoresis (Comet) assay. Environ. Mol. Mutagen 40, 50–56. Kiskinis, E., Suter, W., Hartmann, A., 2002. High-throughput comet assay using 96-well plates. Mutagenesis 17, 37-43. Knopper, L.D., Mineau, P., McNamee, J.P., Lean, D.R., 2005. Use of comet and micronucleus assays to measure genotoxicity in meadow voles (Microtus pennsylvanicus) living in golf course ecosystems exposed to pesticides. Ecotoxicology 14, 323-335. Kohner, E.M., Stratton, I.M., Aldington, S.J., Turner, R.C., Matthews, D.R., 1999. Microaneurysms in the development of diabetic retinopathy (UKPDS 42). UK Prospective Diabetes Study Group. Diabetologia 42, 1107–1112. Kothari, V., Stevens, R.J., Adler, A.I., Stratton, I.M., Manley, S.E., Neil, H.A., Holman, R.R., 2002. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke 33, 1776–1781. Kumaravel, T.S., Jha, A.N., 2006. Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat. Res. 605, 7-16. Lotti, M., 2001. Clinical toxicology of anticholinesterase agents in humans. In: Handbook of Pesticide Toxicology. Krieger, R.I., ed. 2nd edition, San Diego 2, 1043-1085. Mahjoubi- Samet, A., Fetoui, H., Zeghal, N., 2008. Nephrotoxicity induced by dimethoate in adult rats and their suckling pups. Pestic. Biochem. Physiol. 91, 96-103. Nwani, C.D., Nwachi, D.A., Okogwu, O.I., Ude E.F., Odoh, G.E., 2010. Heavy metals in fish species from lotic freshwater ecosystem at Afikpo, Nigeria. J. Environ. Biol. 31, 595-601. Olive, P.L., Banath, J.P., Durand, R.E., 1990. Heterogeneity in radiation–induced DNA damage and repair in tumor and normal cells measured using the "comet" assay. Radiat. Res. 122, 86–94. Parkash, J., 2016. Effect of Endosulphan and Dimethoate Pesticides on Haematological Parameters of Fresh Water Fish Channa punctatus. RRJZS 4, 8 – 33. Sasaki, Y.F., Izumiyama, F., Nishidate, E., Matsusaka, N., Tsuda, S., 1997. Detection of rodent liver carcinog.en genotoxicity by the alkaline single cell gel electrophoresis (Comet) assay in multiple mouse organs (liver, lung, spleen, kidney and bone marrow). Mutat. Res. 391, 201-214. Sayim, F., 2007. Dimethoate-induced biochemical and histopathological changes in the liver of rats. Exp. Toxicol. Pathol. 59, 237-243. Schabath, M.B., Spitz, M.R., Grossman, H.B., Zhang, K., Dinney, C.P., Zheng, P.J., Wu, X., 2003. Genetic instability in bladder cancer assessed by the Comet assay. J. Natl. Cancer Inst. 95, 540–547. Sharma, Y., Bashir, S., Irshad, M., Nagc, T.C., Dogra, T.D., 2005. Dimethoate-induced effects on antioxidant status of liver and brain of rats following subchronic exposure. Toxicology 215, 173-81. Singh, N.P., McCoy, M.T., Tice, R.R., Schneider, E.L., 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175, 184-91. Tice, R.R., Agurell, E., Anderson, D., Burlinson, B., Hartmann, A., Kobayashi, H., Miyamae, Y., Rojas, E., Ryu, J.C., Sasaki, Y.F., 2000. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen 35, 206-221. Tsuda, S., Kosaka, Y., Matsusaka, N., Sasaki, Y.F., 1998. Detection of pyrimethamine-induced DNA damage in mouse embryo and maternal organs by the modified alkaline single cell gel electrophoresis assay. Mutat. Res. 415, 69-77. Undeger, U., Basaran, N., 2005. Effects of pesticides on human peripheral lymphocytes in vitro: induction of DNA damage. Arch. Toxicol. 79, 169-176. US Environmental Protection Agency, 1988. Fact Sheet Number 171, Karate (PP321). Washington, DC. Ventura, B.C., Angelis, D.F., Marin Morales, M.A., 2008. Mutagenic and genotoxic effects of the Atrazine herbicide in Oreochromis niloticus (Perciformes, cichlidae) detected by the micronuclei test and the comet assay. Pestic. Biochem. Physiol. 90, 42–51. WHO (World Health Organization), 1990. Cyhalothrin, Environmental Health Criteria, 99, Geneva, Switzerland. Yahia, D., Ali, F.M., 2018. Assessment of neuro-hepatic DNA damage in male Sprague–Dawley rats exposed to organophosphates and pyrethroid insecticides. Environ. Sci. Pollut. Res. 25, 15616–15629. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||