|
|
Journal of Advanced Veterinary Research Volume 9, Issue 3, 2019, Pages: 81-90 www.advetresearch.com |
|
|
Fraudulence Risk Strategic Assessment of Processed Meat Products |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Doaa S. Abdel-Maguid1, Rania S. Zaki2, Soha A. Soliman3, Hanan H. Abd-Elhafeez4, Sotohy A. Mohamed5 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1Department of Forensic and Toxicology, Faculty of Veterinary Medicine, New Valley University, Egypt. 2Department of Food Hygiene, Faculty of Veterinary Medicine, New Valley University, Egypt. 3Department of Histology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt. 4Department of Anatomy, Embryology and Histology, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt. 5Department of Animal and Environmental Hygiene, Faculty of Veterinary Medicine, Assiut University, Egypt. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Received: 10 June 2019, Accepted: 25 June 2019 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Corresponding author: Doaa S. Abdel-Maguid (Safwat_doaa79@yahoo.com) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A total of 450 samples of different meat products (luncheon chicken, luncheon meat, sausage, beef burger, minced meat, and kofta) were examined. Fifty samples of each type of product were collected from different supermarkets in Assiut City. All of the samples were analysed by different microscopy techniques (light, fluorescence, histochemical microscopy, and scanning electron microscopy (SEM)) for the detection of meat adulteration. Haematoxylin-eosin (HE) staining was used for general histological examinations. Different histochemical techniques were used to stain paraffinised sections. The adulterated tissues detected were the nuchal ligament, large elastic blood vessels, muscular artery, elastic fibers, lung, cardiac muscle fibers, tendon, spongy bone, bone of immature animals, adipose tissue, cartilage (hyaline and white fibrocartilage), and smooth muscle of visceral organs. SEM detected contamination of the minced meat by bacteria and yeast. Fluorescence microscopy was used as an effective method for the detection of bone and cartilage. Interestingly, the stained acidophilic cytoplasm of skeletal muscle changed to basophilic, and the skeletal muscle was suspected to be diseased. The findings of the present work provide qualitative evaluations of the detection of unauthorised tissues in different meat products using different effective histological techniques. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Keywords: Meat products, adulteration, Histochemical, fluorescent, scanning electron microscopic examination. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Introduction |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Meat is an abundant source of different essential nutrients, such as vitamins, proteins and minerals (Binnie et al., 2014). More than half of commercialised meat incorporates heated, cooked, raw and processed meat products (Dayyani et al., 1998). There are myriad of meat products with ingredients that are not included in the labels, which is considered fraud or food adulteration (Ballin, 2010). Food adulteration is an issue of crucial concern in many countries not only because of the safety hazards involved but also for economic imputations. Food adulteration is used to increase the weight of the food or improve its appearance but decreases its quality. Food adulteration is done without the assent and awareness of the consumer (U.S. Law). One type of adulteration is the use of animal tissues, such as central nervous tissues, which is considered a major problem in the transmission of infectious agents to humans (Herde et al., 2005). The usage of bovine brain tissues, spinal cord, eyes and tonsils as a raw substance in meat products has been banned in the European Union since October 2000. These banned tissues are considered "specified risk material (SRM)” and should be burnt (Tersteeg et al., 2002). Furthermore, some meat products have plant-based additives, which can be considered allergens (Pospiech et al., 2009). Another kind of adulteration is the injection or addition of water or an aqueous solution to the meat, which aims to increase its bulk weight (Neto et al., 2016). The next type of food product adulteration is the addition of various flavours to mask the presence of media needed by microorganisms to perform their actions; however, this addition increases the toughness of meat. Accordingly, meat is considered a suitable environment for the growth of many types of microorganisms due to its high water content and conductive pH. Many undesired ingredients may be added to meat products such as sausage and luncheon meat to reduce their price or cost of processing (Lumley, 1996). Apart from being involved in adulteration, some animal tissues, such as the brain and spinal cord, could be a source of infectious agents and cause problems, such as bovine spongiform encephalopathy (BSE) in human (Herde et al., 2005). The quality of meat products, especially sausage, is closely related to the amount of both skeletal muscles and connective tissue and could be decreased by the latter (Ghisleni et al., 2010). Food authentication and adulteration are serious matters in the meat industry and have raised the level of standards (Batt et al., 2015). An indispensable element to determine the quality of food products is the measurement of quality parameters using appropriate methods and techniques. The convenience with which the methods and techniques are used in these measurements is important to ensure the information about the quality of the food product (Lyubchyk et al., 2015). The usual methods used for detecting meat fraud are complex laboratory techniques, which are expensive and time consuming. Therefore, a quick forensic method is required (Neto et al., 2016). The preferred methods of quality control have been histological examinations, which have been revealed by many studies to be able to detect fraud in food and meat product (Latorre et al., 2015). Currently, different imaging methods are available for microscopic evaluation of food constituents. The most commonly used imaging methods are light microscopy to study microstructure and electron microscopy, such as scanning electron microscopy (SEM), to study foodstuff ultrastructure (Pospiech et al., 2011). The aim of this work was to detect meat product adulteration and risk ingredients and to evaluate meat quality using different efficient, rapid, easy and economical histological analyses. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Materials and methods |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Sample collection Fifty samples from 6 different meat products (luncheon chicken, luncheon meat, sausage, beef burger, minced meat, and kofta) shown in Table 1 were randomly purchased from different supermarkets from Assiut City, Egypt. Table 1. Quantities of different meat product samples.
Fixation of the samples Twenty-five samples of each meat product were selected for light microscopic examination, and 25 samples were selected for SEM examination. From each sample, three parts of approximately 1x1 cm3 were taken from three different areas. The samples were fixed in Bouin’s solution for 24 hours for light microscopic examination and Karnovsky’s fixative for SEM (Karnovsky, 1965). Sample processing for light microscopic examination The samples fixed by Bouin’s were washed in 70% alcohol for three changes (3 x 24 h) and then dehydrated in an ascending series of ethanol (80% and 90% for 3 hours in each concentration and 100% I and II for one hour of each change). The samples were cleared for one week using methylbenzoate I and II, followed by impregnation in Paraplast paraffin (Sigma Aldrich) (I, II, III for 3 hours at each concentration). Sections of 5-Ü7 μm were serially cut from each paraffin-embedded block by a Richert Leica RM 2125 microtome (Germany) and mounted on glass slides. The sections were kept dry in an incubator at 40°C. Conventional histological and histochemical staining The cut paraffin sections were stained with haematoxylin-eosin (HE) (Harris, 1898), which is used for general histological examinations. When the routine HE-stained tissue sections were used, various structures could not be identified; therefore, special stains were used to confirm the histochemical reactivity of the complex structure. We used different special stains to differentiate between the various structures: Crossman’s trichrome (Crossman, 1937) and Van Gieson’s (Van Gieson, 1889) stains, both of which are connective tissue stains and were used to distinguish between the connective tissue fiber and muscle tissue in different organs. The connective tissue is stained green, and the muscle is stained red. Verhoeff’s-Van Gieson (VVG) stain was used to detect the presence or absence of elastic fibers in tissue sections of interest. The elastic fibers and cell nuclei were stained black by the Verhoeff component of the VVG stain. The collagen and muscle that had an affinity for the Van Gieson counter-stain were stained red, and the cell cytoplasm and other tissue components were stained yellow. Alcian blue pH 2.5-periodic acid Schiff (AB pH 2.5 / PAS) was used to stain the neutral and acidic polysaccharide structures. Bromophenol blue (Pearse, 1960) was used as a specific stain of total protein structure, which appeared blue. The conventional histological and histochemical stains were cited by Bancroft et al. (2013). The stained sections were examined using a Leitz Dialux 20 microscope. Photographs were taken using a Canon digital camera (Candison Powershot A95). Fluorescent staining of cut paraffin sections Acridine orange (AO) was used as a simple fluorescent stain for demonstrating different structures, such as cartilage, growing bone and large-sized arteries. Using the AO stain The procedure according to (Hoff et al., 1985) was used with modification. Stock solution (0.5%): 50 mg of AO was dissolved in 10 ml of distilled water (DW) and stored in the refrigerator. Staining solution: 1 ml of AO stock solution and 0.5 ml of glacial acetic acid were added to 50 ml of DW. The pH of the solution was approximately 3, and the AO concentration was 0.01%. Staining procedure: 5-μm paraffin sections were dewaxed (2 times for 30 minutes) and rehydrated in a descending series of ethanol (100, 95, and 70%) and DW. The dried tissue sections on glass slides were fixed with methanol and dried in a trough with an AO staining solution (0.01%). After 2 minutes of staining, the slides were washed gently with DW and dried. The sections were then analysed using a Leitz DM 2500 microscope with an external fluorescent unit (Leica EL 6000). Sample processing for SEM Three representative specimens from each sample were washed several times in pH 7.2phosphate buffer and then fixed in Karnovsky’s fixative at 4°C for 24 hours. Thereafter, the specimens were processed according to the description of Abdel Hafeez et al. (2016). Finally, the specimens were coated with gold using a JEOL -1100 E-ion sputtering device and observed with a JEOL SEM (JSM – 5400 LV) at 10 kV at the Electron Microscopy Unit of Assiut University. SEM were used to observe bacteria, either cocci or bacilli, fungi and food additive crystals in addition to other structures. Digitally colouring the SEM images SEM images were digitally coloured using the Photo Filter 6.3.2 program to identify the detailed structure of different tissues and organs on the same electron micrograph. The methods used were developed by Abdel Hafeez et al. (2016); Abdel Hafeez and Soliman (2016 and 2017); Soliman and Abdel Hafeez (2016) and Soliman et al. (2017). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Results |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
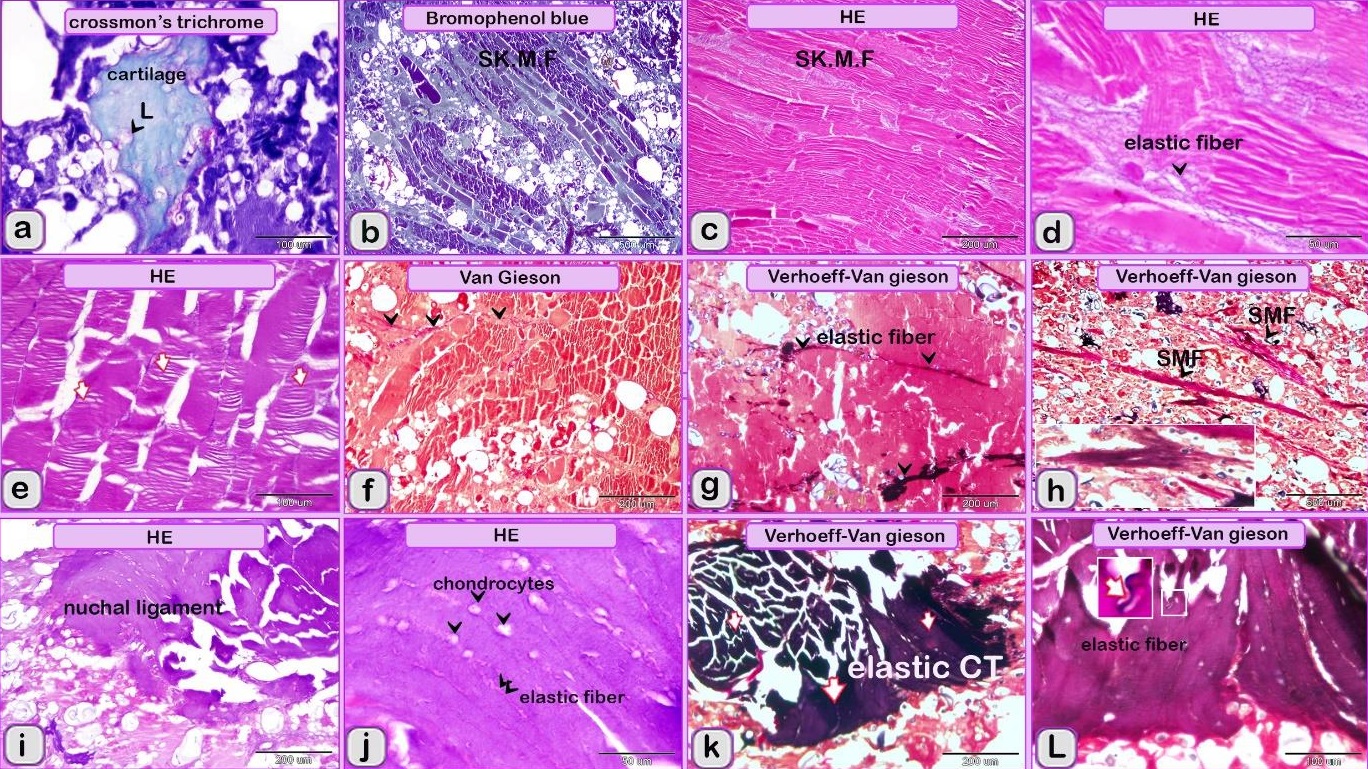
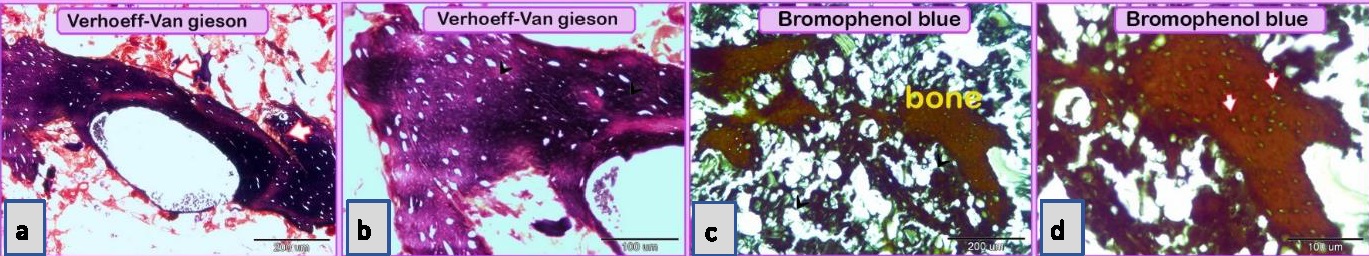
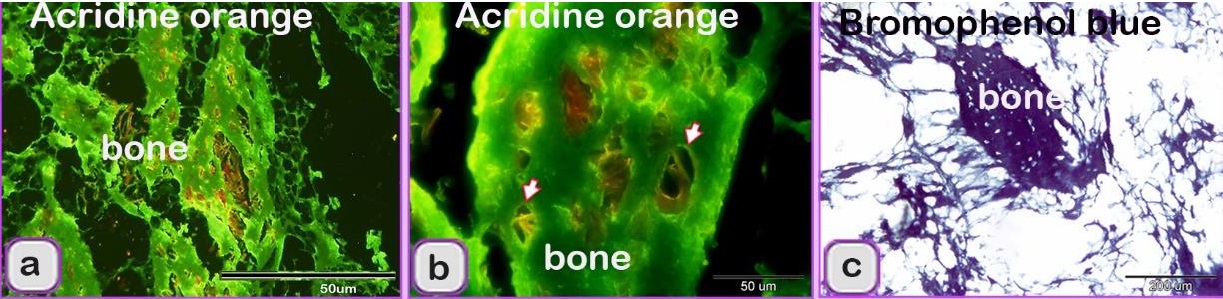
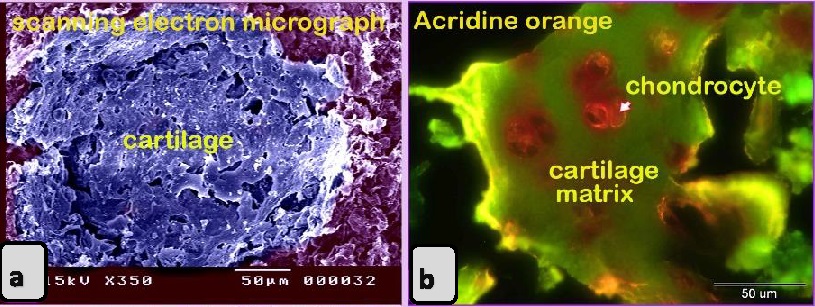
Using specific stains, the articular cartilage and growth plate contained proteoglycan. The hyaline cartilage matrix was stained green by Crossman's trichrome and blue by AB pH 2.5/PAS. White fibrocartilage and elastic fibers were stained dark blue or black, respectively, by the VVG stain. Bone tissue was shown by bromphenol blue as a matrix containing protein stained purple by the AB pH 2.5 /PAS stain, black by the VVG stain, while red by Van Gieson’s. The nuchal ligament was confirmed by the VVG stain. Additionally, the collagen structure was stained. The smooth muscle fibers of visceral organs were stained red by Crossman’s trichrome and Van Gieson’s stains. The elastic membranes of large or elastic blood vessels and muscular or medium-sized arteries were stained black by the VVG stain. Tendon or fascia (collagenous fibers) appeared green with Crossman’s trichrome and orange with Van Gieson’s stain. The adipose connective tissue or fat cells were stained by Crossman’s trichrome stain, Van Gieson’s stain and bromophenol blue. The nerve trunk was stained blue with bromphenol blue. The crystals of food additives were demonstrated by different stains, such as Van Gieson’s stain, and were stained brown with bromphenol blue.
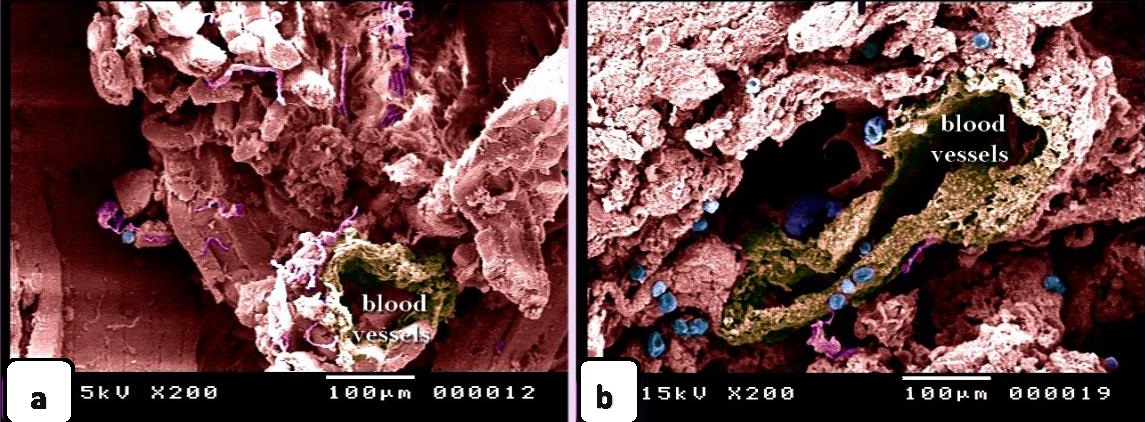
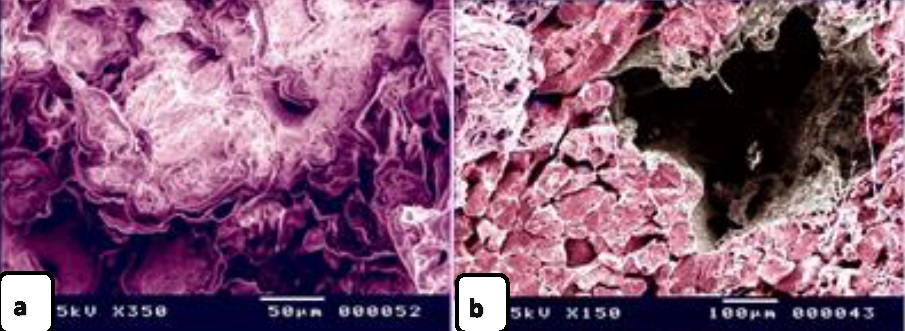
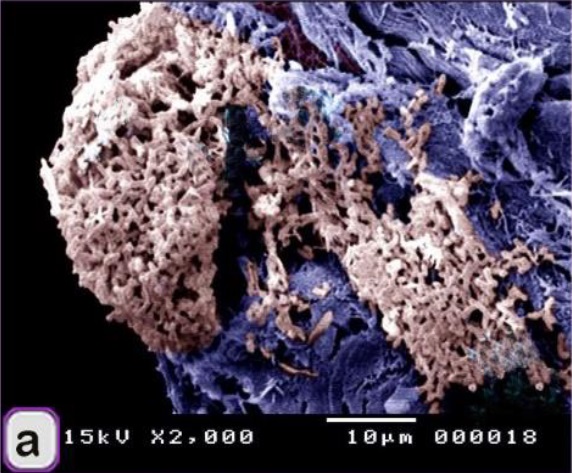
Fig. 1. Paraffin sections showing adulteration of the luncheon meat a: The meat samples contained cartilage. The cartilage matrix, which was stained green and identified by the presence of lacunae (L, arrowhead), contained chondrocytes. b, c, d: The skeletal muscles (SK.M. F) exhibited signs of degeneration, such as loss of the nucleus and myofibrils. Note the elastic fiber: The skeletal muscle fibers stained by HE were basophilic (arrowheads), which may indicate skeletal disorders such as myopathy. f,g: The degenerated muscle fibers that were recognized by the loss of cross-striations. Note the elastic fibers (arrowheads). h: The smooth muscle fibers of the visceral organs were identified by the characteristic spindle-shaped muscle cells that had centrally located nuclei. i, j: A part of the nuchal ligament that was identified by the elastic fibers (double arrow) and the fibrocartilage in which the chondrocytes (arrowheads) were arranged in rows. k, l: A part of the nuchal ligament that was identified by the elastic connective tissue that stained positive for VVG. Note the elastic fibers (arrow). Meat luncheon contained cartilage. The cartilage matrix, which was stained green by Crossman's, was identified by the presence of lacunae that contained the chondrocytes (Fig. 1a). The skeletal muscles exhibited signs of degeneration, such as loss of the nucleus, myofibrils (Fig. 1b,c) and elastic fibers (Fig. 1d). These skeletal muscle fibers stained basophilic by HE, which may indicate skeletal disorders such as myopathy (Fig. 1e). The meat sample containing elastic fibers appeared black by the VVG stain, and degenerated muscle fibers were recognized by the loss of cross-striations (Fig. 1 f,g). The meat sample (red by Van Gieson’s stain), which was adulterated by the smooth muscle fibers of visceral organs, and was identified by the characteristic spindle-shaped muscle cells with centrally located nuclei (Fig. 1 h). A part of the nuchal ligament was identified by the elastic fibers and fibrocartilage in which the chondrocytes were arranged in rows (Fig. 1i,j). This identification was confirmed by the VVG stain, which stained the elastic connective tissue black (Fig. 1 k, l). Bone tissue was identified in the meat sample by the presence of osteocytes located in the lacunae (Fig. 2c, d), the sample appears black by VVG stain (Fig. 2a, b). The unauthorised tissue found in the scanned samples of meat luncheon was nuchal ligament (Fig. 3a), and blood vessels (Fig. 3b).
Fig. 2. Paraffin sections showing adulteration of luncheon meat a, b,c,d: A part of bone tissue, which was recognized by the lacunae in which the osteocytes were located (arrows).
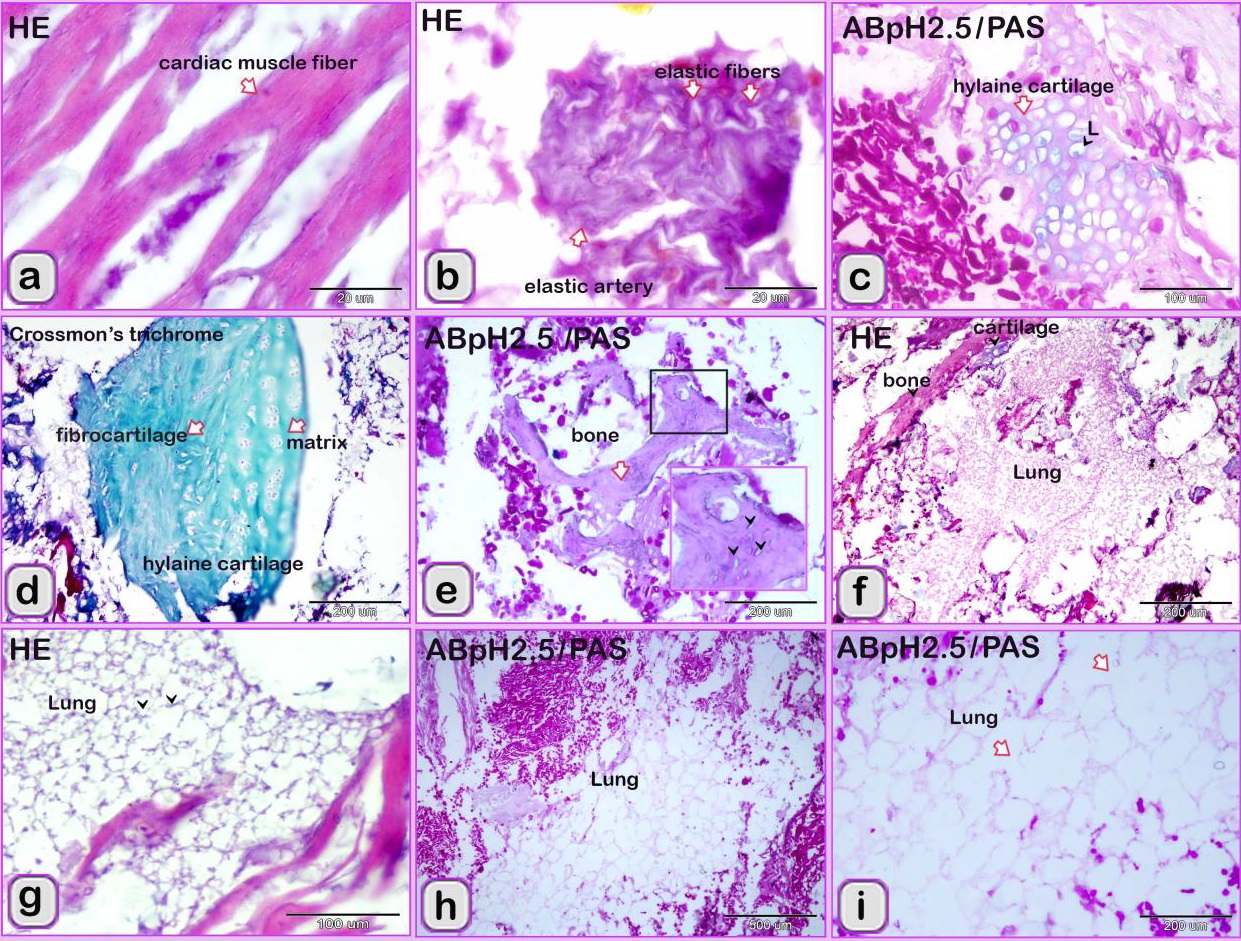
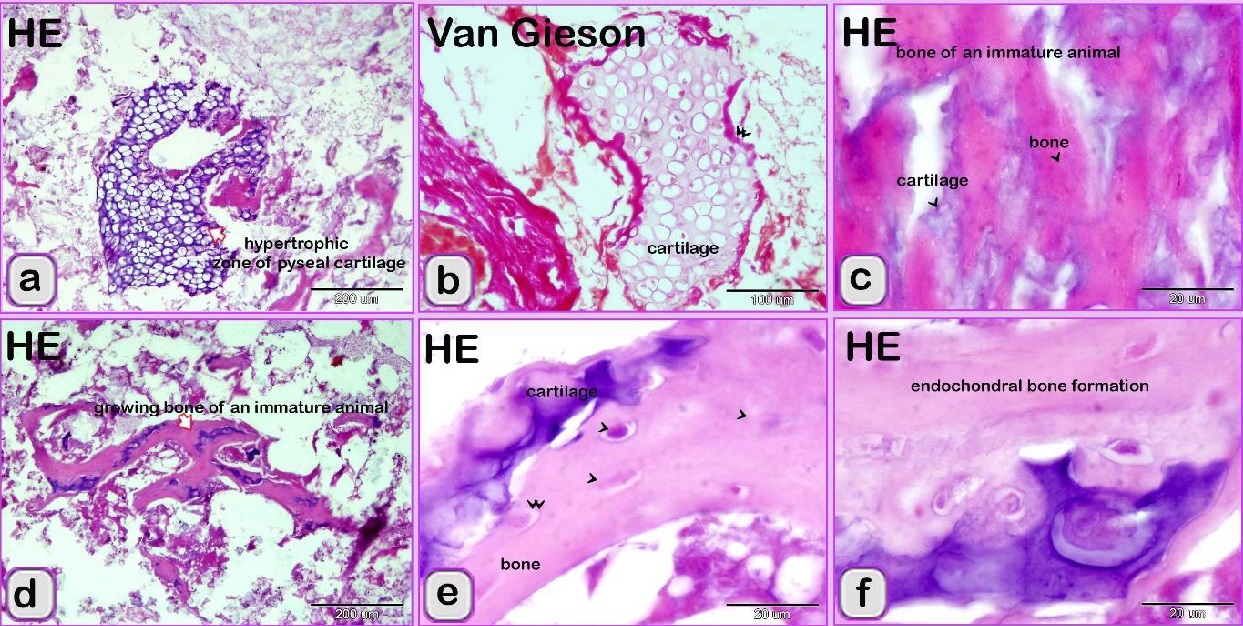
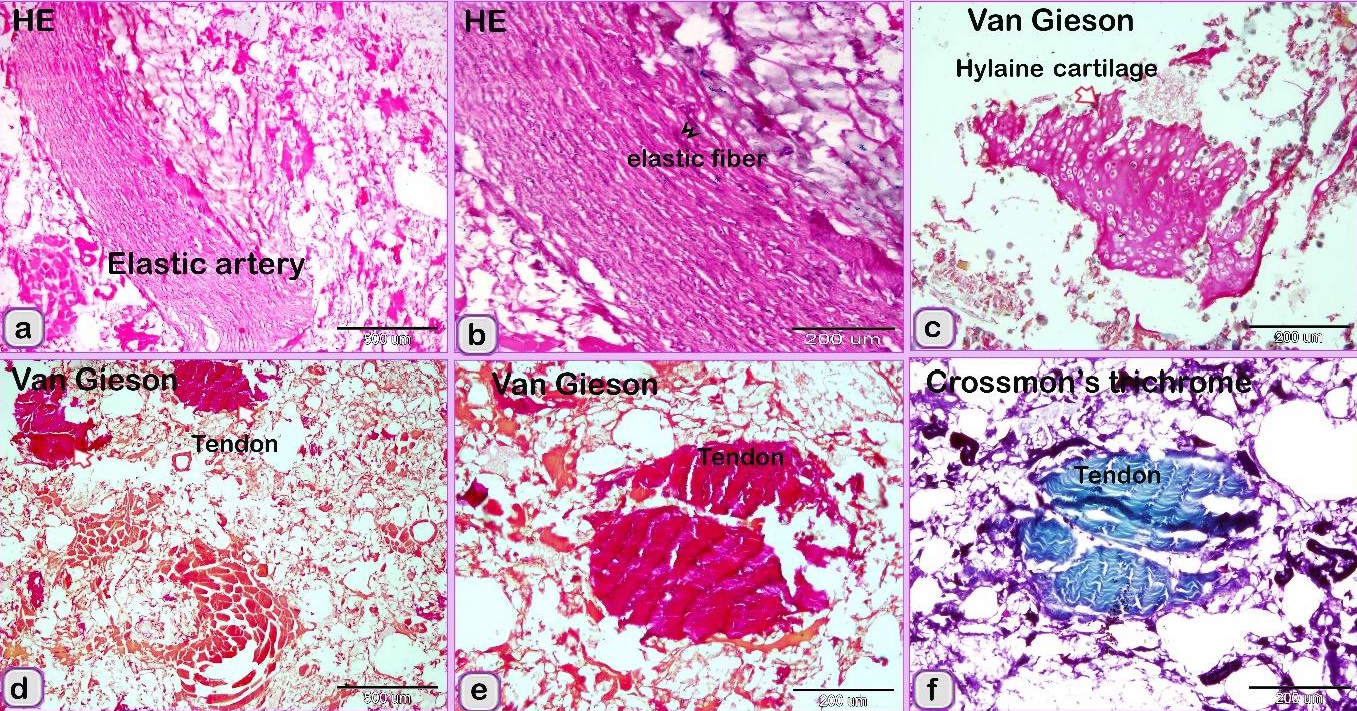
Fig. 3. SEM image showing the adulteration of the luncheon meat a-b: A part of the nuchal ligament, which was identified by fibrocartilage that consisted of collagen fibers (brown coloured) and rows of chondrocytes (blue coloured). a, b: The meat samples contained blood vessels. In luncheon chicken, there were unauthorised animal tissues, including immature and growing long bone, which were identified by the epiphyseal and physeal cartilages. The epiphyseal cartilage was distinguished by randomly organised chondrocytic lacunae. Epiphyseal cartilage was ossified at a secondary ossification centre to form bone tissue. The physeal cartilage was identified by the hypertrophic zone through which endochondral ossification occurred The unsanctioned animal tissue in a beef burger was from heart muscle and was recognized by cardiac muscles that had a branched architecture (Fig. 4a). The meat samples were adulterated by elastic artery, which was recognized by the elastic fibers stained purple with the HE stain (Fig. 4b) and a part of hyaline cartilage, which was identified by chondrocytes located in the lacunae (arrowhead) and embedded in the cartilage matrix stained blue by AB pH 2.5 / PAS and PAS stain (Fig. 4c). The cartilage matrix. Spongy bone was stained purple by AB pH 2.5 / PAS and was perceived by haematopoietic spaces and bone that contained osteocytes in the lacunae (Fig. 4e). Lung tissue was identified by its alveolar architecture (Fig. 4 f-i). A part of the growth plate of a growing long bone of an immature animal in which the hypertrophic zone of the growth plate underwent endochondral bone formation was identified (Fig. 5a, b). A part of growing bone of an immature animal. Endochondral bone formation is a mechanism by which cartilage is replaced by bone tissue. Osteocytes were located in the lacunae (Fig. 5 c-f). The cartilage matrix exhibited a basophilic affinity, while the bone matrix had an acidophilic affinity for the HE stain.
Fig. 4. Paraffin sections showing the adulteration of the beef burger. a: A part of the heart, which was recognized by cardiac muscles that had branched architecture. b: The meat samples were adulterated by an elastic artery, which was recognized by the elastic fibers. c: A part of hyaline cartilage, which was identified by chondrocytes located in the lacunae (arrowhead) and embedded in the cartilage matrix (arrow). d: A part of the articular hyaline cartilage. e: Spongy bone, which was recognized by haematopoietic spaces and bone trabeculae (arrow) that contained osteocytes located in the lacunae (arrow head). f-i: The meat samples were adulterated by lung tissue, which was identified by the alveolar architecture (arrowheads).
Fig. 5. Paraffin sections showing the adulteration of the beef burger a,b: A part of the growth plate of a growing long bone of an immature animal. Note that the hypertrophic zone (arrow) of the growth plate underwent endochondral bone formation (arrowheads). c-f: A part of the growing bone of an immature animal. The endochondral bone formation is a mechanism by which the cartilage is replaced by bone tissue (arrow). Note the osteocytes located in the lacunae (arrowheads).
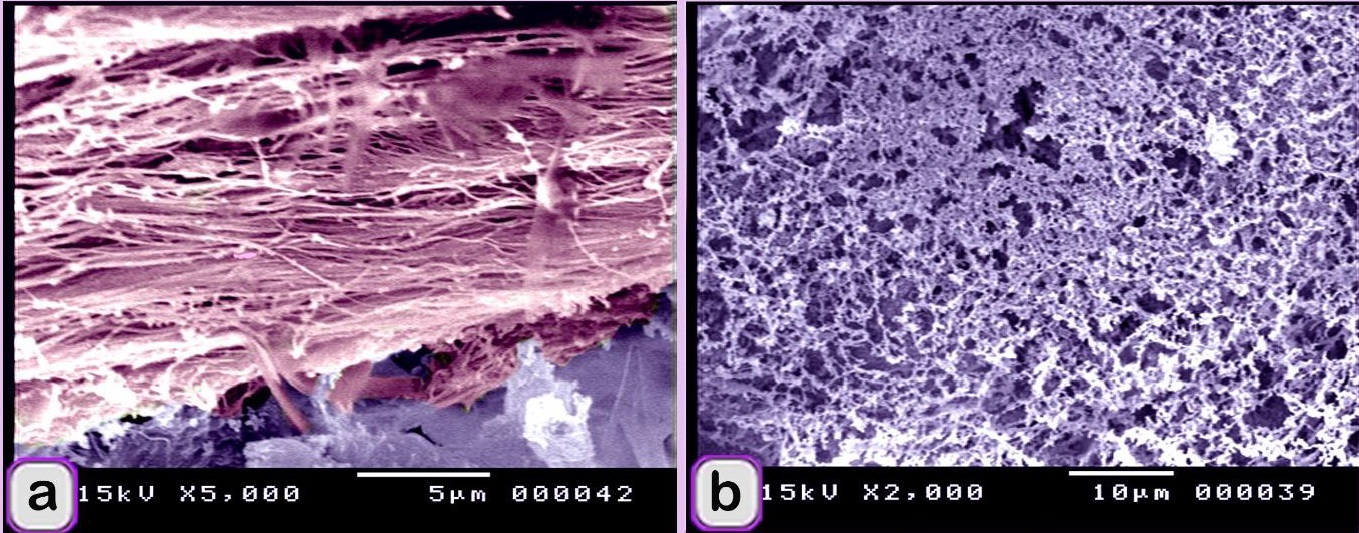
Fig. 6. SEM image showing the adulteration of the beef burger A: The elastic tissue (pink coloured) was identified by elastic fibers that have branched features. b: The meat samples were adulterated by lung tissue, which was recognized by the alveolar architecture (violet coloured). The meat sample contained large sized artery or elastic artery (Fig. 7 a, b) and a part of hyaline cartilage, which was identified by the presence of chondrocytes embedded in the cartilage matrix and was stained red by Van Gieson’s stain (Fig. 7c). A portion of a tendon was distinguished by regularly arranged collagen fibers stained red by Van Gieson’s stain and green by Crossman’s trichrome stain (Fig. 7 d-f). fat tissue was identified. Degenerated skeletal muscles were identified by the loss of myofibril cross-striations and nuclei. These observations confirmed that the scanned samples were adulterated by animal tissue. The meat samples were adulterated by elastic tissue (violet coloured) and skeletal muscle (Fig. 8a). The skeletal muscle had very distinct myofibrils (Fig. 8b) surrounded by collagen fibers (Fig.8 c). Fat cells (Fig.9 a), and skeletal muscle that contained a large vein (Fig. 9b).
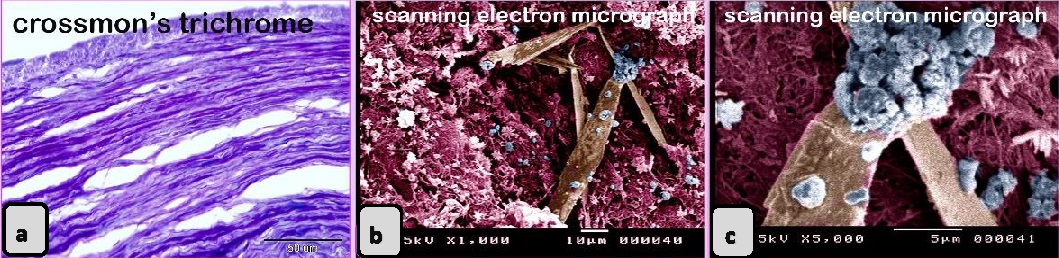
Fig. 7. Paraffin sections showing the adulteration of the beef burger. a, b: The meat sample was adulterated by a large artery or elastic artery, which was recognized by the elastic fibers (double arrowhead) predominating in the tunica media. c: The meat sample contained a part of the hyaline cartilage (arrow), which was identified by the presence of chondrocytes embedded in the cartilage matrix. d-f: A part of a tendon was distinguished by the regularly arranged collagen fibers.
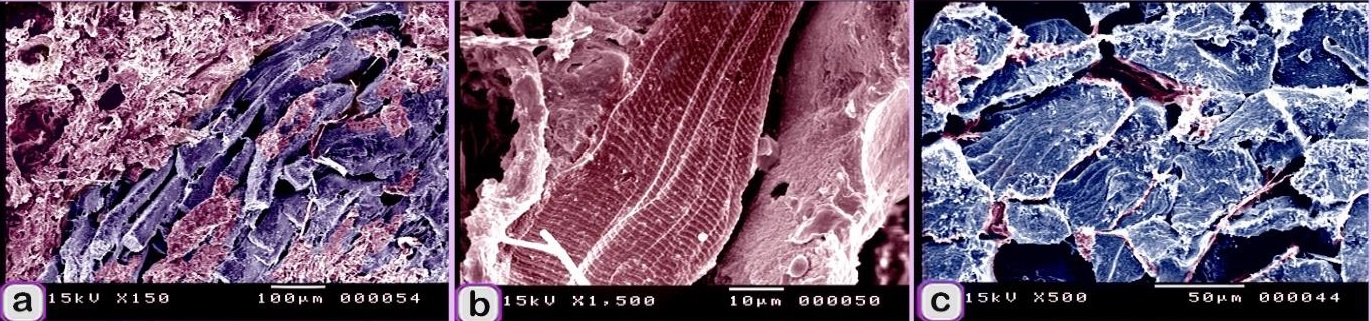
Fig. 8. Digitally colored scanned electron micrograph showing the adulteration of beef burger. A: meat samples were adulterated by elastic tissue (violet colored), skeletal muscle (red colored). B: skeletal muscle (red colored) had well-distinct myofibrils. C: skeletal muscle (blue colored) surrounded by collagen fibers (pink colored).
Fig. 9. Digitally colored scanned electron micrograph showing the adulteration of beef burger with animal tissue. a: The meat samples were adulterated by fat cells (Pink colored). b: meat samples were adulterated by skeletal muscle (red colored) that contained Large vein (brown color). The scanned meat samples of the beef burger were confirmed to be adulterated by animal tissues, such as elastic tissue, which was identified by elastic fibers with branched features (Fig. 6a), and lung tissue, which was identified by its alveolar architecture (Fig. 6b). The sausage by an elastic artery. A part of the nuchal ligament, which contained elastic tissue and fibrocartilage, was identified. The fibrocartilage was recognized by the alignment of chondrocytes in rows. Degenerated fibrocartilage, which exhibited degeneration of the cartilage matrix, was found. The meat samples were adulterated by elastic tissue and collagenous connective tissue with an irregular shape that is characteristic of the capsule of parenchymatous organs. A part of a skeleton was identified. Bone tissue was recognized by bone cells and haematopoietic cells (Fig. 10a), and osteocytes were found in the lacunae (Fig. 10b). The meat sample contained bone, which was stained blue with bromophenol blue (Fig. 10c).
Fig.10. Paraffin sections showing the adulteration of sausage with animal tissue.a-c: The bone tissue of spongy type that was identified by hemopoietic spaces (arrows). Meat containing fibrocartilage, Spongy bone tissue, & Hyaline cartilage. The scanned meat samples of sausage were confirmed to be adulterated by animal tissues. A part of a skeleton was identified. Bone tissue was recognized by bone cells, haematopoietic cells, and osteocytes in the lacunae, the cartilage was identified by chondrocytes located inside the lacuna and surrounded by cartilage matrix (Fig.11 a,b) and a nerve trunk that contained several axons (Fig. 11 c,d).
Fig. 11. Paraffin sections showing the adulteration of sausage a-b: meat samples were adulterated by nerve trunk (arrows) that contained several axons. Minced meat was adulterated with animal tissue. The meat samples were adulterated by adipose tissue (fat) and collagenous connective tissue. The meat samples contained degenerated skeletal muscles that had lost the myofibril cross-striations, collagenous connective tissue (tendon), and degenerated skeletal muscles that had lost the myofibril cross-striations and nuclei. Additionally, the meat samples contained a muscular artery, a part of the nuchal ligament with notable zigzag-shaped fibers, was present, indicating the presence of elastic fibers and a wall of visceral organ was recognized by a thick layer of smooth muscle fibers (Fig. 12a).
Fig. 12. Paraffin sections showing the adulteration of minced meat. a: A wall of the visceral organ. Note the thick layer of smooth muscle fibers. b, c: digitally colored scanned electron micrograph showing fungal contamination (blue colored). The scanned minced meat samples were contaminated by fungi (Fig 12 b,c). Bacteria and yeast contamination was declared in also were adulterated by fibrous tissue (Fig. 13a).
Fig. 13. Digitally colored scanned electron micrograph showing the adulteration of minced meat a: Meat sample contaminated by bacteria and yeast (brown colored) and adulterated by fibrous tissue (violet colored). Kofta from showed evidence of adulteration by degenerated skeletal muscle that had lost the cross-striation. elastic tissue that could be a part of the nuchal ligament, adipose connective tissue and spongy bone tissue identified by haematopoietic spaces. The cardiac muscles were recognized by their branching architecture. Table 2. The percentage ABC Song | Kids Songs and Nursery Rhymes e of unauthorised tissues according to the whole number of samples from the meat products.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Discussion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The diversity of the finished meat products in the consumer market in Egypt is quite great, but a large number of products vary in quality. As such, quality control of meat and meat products is very important (lyubchyk et al., 2015). Manufacturers have a duty to make certain that the meat product quantity is in agreement with the requirements of regulation EC No. 853/2004/10) (Malakauskienė et al., 2016). Recently, the single most troublesome issue that has become visible worldwide is meat adulteration (Sadeghinezhad et al., 2015), which violates food safety, health regulations and religious beliefs. In addition to the alarming increase in food authentication within the last few decades, there has been a noticeable frequency in false labelling and undeclared utilisation of food additives or other contents to replace the skeletal muscles in meat products to earn economic benefits (Orduna et al., 2015). Accordingly, precise methods can be utilised to quantitatively and qualitatively analyse the ingredients in processed meat products to solve the authentication problem in the meat industry. Microbiological and chemical methods alone could not be used to assess the properties of meat products. Depending on the histology of the sample, histological techniques could be used to directly identify other tissues in addition to meat and changes in the structure of meat (Sadeghinezhad et al., 2016). Therefore, research tools are needed to judge the quality or quantity of meat products. The meat products analysed in this research are popular in Egypt, as shown by the regular increase in their manufacturing and selling capacity over time (Latorre et al., 2015). Consequently, this study assessed the quality of these popular meat products by detecting the presence of unpermitted tissues in different foods available to the public in Egypt (Lyubchyk et al., 2015). The composition of the products examined in this study showed that all the suppliers of these meat products did not completely honour the standards of hygiene, food adjustment and products of high quality (Tremlova and Štarha, 2003). The presence of unallowed tissues that were combined with minced meat, sausages, burgers, koftas of beef and luncheon meat of beef and chicken was revealed by image analysis. Those unallowed tissues included skeletal muscles, cartilage, connective tissue, bone, nerve fibers, adipose tissue, growing bone, nuchal ligament, smooth muscles, alveolar elastic tissue and fibrous tissue. Furthermore, the minced meat was contaminated with different types of bacteria and fungi. All the unauthorised tissues, whether edible or inedible, could cause a risk to humans. For these reasons, the criteria established by the International Commission on Microbiological Specifications for Foods (ICMSF) have been extensively used for estimating the hygienic quality of edible offals (Roberts et al., 1996). All contaminations could be a major cause of food poisoning in humans (Scallan et al., 2011). Various studies have evaluated unauthorised tissues in meat products. In agreement with results from the present study, according to the histological evaluation performed in the USA (Prayson et al., 2008b), adipose tissues, connective tissue, cartilage, blood vessels, and bone and peripheral nerves were observed. Dayyani et al. (1998) detected histologically smooth muscles in meat products. Latorre et al. (2015) detected different ratios of unpermitted tissues, including cartilage, heart muscles, bone, spleen, lymph node and oesphagus, in different sausage samples from Iran. Another study (Sepehri Erayi 2008) showed unauthorised tissues as nerves, cartilages, blood vessels and adipose tissues. The results of the present study reported the presence of nuchal ligament in samples of luncheon meat, sausage plus kofta, agreed with those of Dayyani et al. (1998) who identified nuchal ligament in a heated Iranian sausage, revealing the utilisation of meat received (gained) from a butchered animal's head in processed meat. They also reported the presence of the salivary gland, which is in the head; however, it could not be detected in the current study. We suggest that this difference is due to the fact that sections were taken randomly from the used organ. Brain tissue could carry infectious agents that might be transferred to consumers (Abdel Hafeez et al., 2016). Moreover, the present study showed the presence of a nerve trunk in sausage. One of the important views on forbidden tissues, such as the spinal cord and brain, is that they can hold infectious agents that are harmful to consumers (Herde et al., 2005). In addition to a rigorous hygiene examination, other new issues regarding ready-to-eat meat require attention. Bovine spongiform encephalopathy (BSE) belongs to a family of diseases known as transmissible spongiform encephalopathies (TSEs), which were developed from the build-up of abnormal prion proteins in the brain and central nervous system. TSE causes a variant form of Creutzfeldt-Jacob disease (vCJD), which leads to death in humans. As a result of this disease, certain tissues of cattle (spleen, tonsils, intestine, mesentery, spinal cord and full head) that are uttermost likely to contain the BSE agent are categorised as SRMs and must be excluded and destroyed to avoid involving either humans or animal food chains (Toldra et al.,2012). Recently, the Food and Drug Administration (FDA, 2004) declared rules to avoid the spread and establishment of BSE in the United States, inclusive of the injunction on the use of high-risk cattle-derived material that could hold SRMs. The materials are the skull, brain, spinal cord, eye, terminal ganglia, vertebral column at the root of ganglia, vertebral column, spinal cord, distal ileum and small intestine of all cattle (FDA, 2004). None of the examined samples were adulterated with hollow organs, an observation that is not in agreement with the study by Abdel Hafeeze et al. (2016). Ince and Ozfiliz (2018) stated that alveolar tissues were detected in Turkish-type sausage samples. However, this study differs from that of Ayaz et al. (2007) in that no alveolar structures were found in the lung; only bronchi structures were detected, and this discrepancy revealed differences caused by random sampling. The smooth muscle fibers of visceral organs were detected in the luncheon meat samples. The degenerative muscle fibers of the heart were detected in the kofta samples, these findings are in accordance with those of Inal (1992), who reported that the muscle cells of intestinal mucosa and heart muscles were detected in salami and sausage samples. The obtained results showed that immature or growing long bone was present in samples of luncheon chicken and beef burger, indicating that these meat products were adulterated by dead foetuses. Foetal flesh is not allowed in meat due to its soggy appearance and low quality. Additionally, it results from abortions that may be caused by diseases and could severely contaminate the food supply and infect the consumers (Abdel Hafeez et al., 2016). The appearance of bone tissue in the luncheon meat, beef burger, sausage and kofta samples (all the sample types, except luncheon chicken) was frequent, as mentioned by Tremlova and Starha (2003), who quantified bone tissues in meat products by image analysis. Bone pieces do not ordinarily exist in meat products, so their presence is uncommon and can reveal the trouble of minimally processed meat material (Pospiech et al., 2011), which truly affects the quality of meat products. The skeletal muscle fibers in the luncheon meat indicated skeletal disorders due to different diseases, such as myopathy (Valentine, 2008). The study revealed the existence of degenerative muscle in all type of samples except luncheon chicken. Consumers need to know about processed food and the differences between the 1st type of skeletal muscle (slow contracting, dark fiber) and the 2nd type (fast contracting, light fiber). This knowledge is important because the detection of meat softness depends on the percentage of various fiber types according to the proportion of light and dark fibers (Picard et al., 1998). Additionally, the proportion of light and dark fibers is a fitting metric for adult and foetal meat detection. The skeletal muscle of a bovine foetus contains primarily more dark fibers than light fibers (Crosier et al., 2002). Buche and Manron (1997) evaluated meat quality through image analysis to determine the marbling of meat or to measure the different muscle fiber parameters that are beneficial. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conclusion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
This research approves the use of common histological methods and scanning electron microscope to detect adulteration of processed meat. Histological methods detect adulteration of processed meat by different tissues including nuchal ligament, large elastic blood vessels, muscular artery, elastic fibers, lung, cardiac muscle fibers, tendon, spongy bone, bone of immature animals, adipose tissue, cartilage and smooth muscle of visceral organs. SEM is valuable in detecting contamination by bacteria and yeast. Fluorescence microscopy is an effective method for the detection of adulteration using bone and cartilage. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conflict of Interests |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
References |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abdel-Hafeez H.H., Zaki R.S., Abd El-Maguid D.S., 2016. Applying Light, Histochemical and Scanning Histological Methods for the Detection of Unauthorized Animal and Herbal Content in Street Meat Sandwich: What is in the Sandwich We Eat?. J. Food Process. Technol. 7, 643. Abdel-Hafeez, H.H., Soliman, S.A., 2016. Origin of Rodlet Cells and Mapping Their Distribution in Ruby-Red-Fin Shark (Rainbow Shark) Epalzeorhynchosfrenatum (Teleostei: Cyprinidae): Light, Immunohistochemistry and Ultrastructure Study. J. Cytol. Histol. 7, 435. Abdel-Hafeez, H.H., Soliman, S.A., 2017. New Description of Telocyte Sheaths in the Bovine Uterine Tube: An Immunohistochemical and Scanning Microscopic Study. Cells Tissues Organs 203, 295-315. Ayaz, Y., Oruç, E., Ulusoy, Y., Kaplan, Y.Z., 2007. Experimental study on tissue detection by microscopical examination in minced meat. J. Etlik. Vet. Microbiology 18, 17-20. Ballin, N.Z., 2010. Authentication of meat and meat products. Meat Science 86, 577–587. Bancroft J.D., Layton C., Suvarna K., 2013. Bancroft’s Theory and Practice of Histological Techniques, Churchill Livingstone. Batt, M.M., Jalal, H., Para, P.A., Qadri, K., 2015. Fraudulent adulteration/ substitution of meat: a review. International journal of recent research and applied studies 2 (12), 22-33. Binnie, M.A., Barlow, K., Johnson, V., Harrison, C., 2014. Red meats: time for a paradigm shift in dietary advice. Meat Science 98(3), 445-451. Buche, P., Mauron, D., 1997. Quantitative characterization of muscle fiber by image analysis. Comput. Electr. Agr. 13, 189 – 217. Crossman, G., 1937. A modification of Mallory’s connective tissue stain with discussion of the principle involved. Anat. Rec. 69, 33-38. Crosier, A.E., Farin, C.E., Rodriguez, K.F., Blondin, P., Joseph, E. Alexander, Peter, W.F., 2002. Development of skeletal muscle and expression of candidate genes in bovine fetuses from embryos produced in vivo or in vitro. Biol. Reproduce 67, 401-408. Dayyani Dardashti, A., Rokni, N., Rezaian, M., 1998. Histological and histometrical studies of different heated sausages. World congress foodborne infections and intoxications 2, 1073-1078. FDA, 2004. Interim final rule — Use of materials derived from cattle in human food and cosmetics. Food and Drug Administration 21 CFR parts 189 and 700. Federal Register, 69, 134. Ghisleni, G., Stella, S., Radaelli, E., Mattiello, S., Scanziani, E., 2010. Qualitative evaluation of tortellini meat filling by histology and image analysis. International Journal of Food Science and Technology 45, 265–270. Harris, H.F., 1898. A new method of ripening hematoxylin; in Romeis, B. (ed): Mikroskopi-sche Technik. Munich, Oldenburg. Herde, K., Bergmann, M., Lang, C., Leiser, R., Wenisch, S., 2005. Glial fibrillary acidic protein and myelin basic protein as markers for the immunochemical detection of bovine central nervous tissue in heat-treated meat products. Journal of Food Protection 68, 823–827. Hoff, R.G., Newman, D.E., Staneck, J.L., 1985. Bacteriuria screening by use of acridine orange-stained smears. J. Clin. Microbiol. 21, 513-516. İnal, T., 1992. Besin Hijyeni Hayvansal Gıdaların Sağlık Kontrolü. Final Ofset Publishers, Istanbul. Ince, E., Ozfiliz, N., 2018. Detection of adulterations in fermented and heat-treated Turkish type sausages by histological examination. Ankara Üniv Veteriner Fakültesi dergisi 65, 99-107. Karnovsky, M.J., 1965. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 27, 137A-138A. Latorre, R., Sadeghinezhad, J., Hajimohammadi, B., Izadi, F., Sheibani, M.T., 2015. Application of Morphological Method for Detection of Unauthorized Tissues in Processed Meat Products. Journal of Food Quality and Hazards Control 2, 71-74. Lumley, I.D., 1996. Authenticity of meat and meat products. In: Ashurst P.R., Dennis M.J. (eds) Food Authentication. Springer, Boston, MA, pp. 108-139. Lyubchyk, O., Mykyjchuk, M., Vorobets, M., 2015. Development of Operational Quality Control Method for Meat Products. Journal of Faculty of Food Engineering XIV, 212-217. Malakauskienė, S., Alionienė, I., Džiugienė, D., Babrauskienė, V., Riedel, C., Alter, T., Malakauskas, M., 2016. Histological analysis for quality evaluation of cured meat sausages. Veterinarija Ir Zootechnika (Vet Med Zoot) T. 74 (96). Migaldi, M., Rossi, G., Sgambato, A., Farinetti, A., Mattioli, A.V., 2016. Histological and immunohistochemical analysis of meat-based food preparations. Progress in Nutrition 18(3), 276-282. Neto, J.J., Santos, J.A., Schwartz, W.R., 2016. Meat adulteration detection through digital image analysis of histological cuts using LBP. CoRR, abs/1611.02260. Orduna, A.R., Husby, E., Yang, C.T., Ghosh, D., Beaudry, F., 2015. Assessment of meat authenticity using bioinformatics, targeted peptide biomarkers and high-resolution mass spectrometry. Food Additives & Contaminants Part A 32 (10), 1709-1717. Picard, B., Duris, M.P., Jurie, C., 1998. Classification of bovine muscle fibers by different histochemical techniques. Histochem. J. 30, 473-479. Pearse, A.G.E., 1960. Histochemistry: Theoretical and Applied. Boston, Little, Brown. Pospiech, M., Tremlova, B., Rencova, E., Randulova, Z., 2009. Immunohistochemical detection of soya protein- optimization and verification of the method. Czech Journal of Food Sciences 27, 11-19. Pospiech, M., Lukášková, Z.R., Tremlová, B., Randulová, Z., Bartl, P., 2011. Microscopic methods in food analysis. Maso international brno. 1, 27-34. Prayson, B., McMahon, JT, Prayson, R.A., 2008b. Fast food hamburgers: what are we really eating? Annals of Diagnostic Pathology 12, 406-409. Prayson, BE, McMahon, JT, Prayson, RA., 2008a. Applying morphologic techniques to evaluate hotdogs: what is in the hotdogs we eat? Annals of diagnostic pathology 12 (2), 98-102. Roberts, T.A., Tompkin, R.B., Baird-Parker, A.C., 1996. Microorganisms in foods. In: Microbiological specifications of food pathogens. Chapman & Hall, London. Sadeghinezhad, J., Hajimohammadi, B., Izadi F., Yarmahmoudi, F. Latorre, R., 2015. Evaluation of the Morphologic Method for the Detection of Animal and Herbal Content in Minced Meat. Czech J. Food Sci. 33(6), 564 –569. Sadeghinezhad, J., Izadi, F., Latorre, R., 2016. Review Paper: Application of Histomorphological Method to Assess Meat Products. ASJ. 13 (2), 73-78. Scallan, E., Griffin, P.M., Angulo, F.J., Tauxe, R.V., Hoekstr,a R.M., 2011. Foodborne illness acquired in the United States unspecified agents. Emerg. Infect. Dis. 17, 16-22. Sepehri Eraei, S., 2008. Histological methods evaluation for detection of adulteration of raw meat products supplied in Tehran. DVM thesis. Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran. Soliman, S.A., Abd-Elhafeez, H.H., 2016. Are C-KIT, MMP-9 and Type II Collagen Positive Undifferentiated Cells Involved in Cartilage Growth? A Description of Unusual Interstitial Type of Cartilage Growth. J. Cytol. Histol. 7, 440. Soliman, S.A., Abd-Elhafeez, H.H., Enas, A., 2017. A New Mechanism of Cartilage Growth in mammals “Involvement of CD117 Positive Undifferentiated Cells in Interstitial Growth”. M. J. Cyto. 1(1), 001. Tersteeg, M.H., Koolmees, F.K., 2002. Immunohistochemical detection of brain tissue in heated meat products. Meat Sci. 61, 67–72. Toldra, F., Aristoy, M.C., Mor,a L., Reig, M., 2012. Innovations in value-addition of edible meat by-products. Meat Sci. 92, 290-296. Tremlova, B., Štarha, P., 2003. Histometric Evaluation of Meat Products – Determination of Area and Comparison of Results Obtained by Histology and Chemistry. Czech J. Food Sci. 21, 101–106. U.S. law (21 U.S.C. §342). [Online]. https://www.law.cornell.edu/uscode/text/21/342. Valentine, B.A., 2008. Pathologic findings in equine muscle (excluding polysaccharide storage): A necropsy study. J. Vet. Diagn. Invest. 20, 572–579. Van Gieson, I., 1889. Laboratory notes of technical method for the nervous system. NY Med J 50, 57–60. Wijnker, J.J., Tersteeg, M.H., Berends, B.R., Vernooij, J.C., Koolmees, P.A., 2008. Quantitative histological analysis of bovine small intestines before and after processing into natural sausage casings. J. Food Prot. 71, 1199-204. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||