|
|
Journal of Advanced Veterinary Research Volume 9, Issue 3, 2019, Pages: 107-116 www.advetresearch.com |
|
|
Biochemical Evaluation of some Natural Feed Additives against Dexamethasone-induced Metabolic Alterations in Rabbits |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mohamed A. Kandeil1, Eman T. Mohammed1*, Omaima I. Aly2, Asmaa M. Abd-Elrahman2 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1Biochemistry Department, Faculty of Veterinary Medicine, Beni-Suef University, Egypt. 2Biochemistry Department, Animal Health Research Institute, Giza, Egypt. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Received: 19 June 2019; Accepted: 3 July 2019 *Corresponding author: Eman T. Mohammed (eman.ibrahim@vet.bsu.edu.eg) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Glucocorticoid therapy is limited by numerous metabolic adverse effects associated with long term exposure to excess doses. Therefore, the present study aims to determine the possible protective effects of date palm and/or Saccharomyces cerevisiae probiotics on dexamethasone-induced metabolic changes in rabbits. 25 healthy male white New Zealand rabbits were grouped into group 1 (control), group 2 (2 mg/kg bw/day dexamethasone I/M), group 3 (0.5 g/kg/day date palm flesh+2 mg/kg bw/day dexamethasone I/M), group 4 (1g/kg/day S. cerevisiae probiotic + 2 mg/kg bw/day dexamethasone I/M), group 5 (date palm flesh + S. cerevisiae probiotic + dexamethasone at the aforementioned doses). Dexamethasone injection resulted in marked increases (p≤0.05) in hepatic MDA concentration and catalase activity, as well as significant decreases in hepatic GSH concentration and body weight gain. The serum levels of glucose, lipid profile (TG, cholesterol, VLDL, LDL/HDL risk ratio), and liver function biomarkers (serum total proteins, albumin, |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Keywords: Dexamethasone, Date palm, Probiotics, Rabbits. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Introduction |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Glucocorticosteroids (GCs) are steroid hormones produced by the adrenal cortex. Dexamethasone is one of the potent synthetic glucocorticoids that has a long history of use in veterinary and human medicine for the treatment of a wide range of metabolic diseases and inflammatory disorders (Andrews et al., 1991, Boers et al., 2003). Clinically, dexamethasone is administered for the suppression of inflammation (Czock et al., 2005; Schacke et al., 2002). Long term exposure to high levels of GCs is associated with severe adverse effects and metabolic derangements, including insulin resistance, glucose intolerance (Schacke et al., 2002), hepatic steatosis, central adiposity (Rockall et al., 2003), dyslipidaemia (Wajchenberg, 2000), osteoporosis, cardiovascular disease, Cushing’s syndrome, psychiatric disturbances and immunosuppression (Liu et al., 2013). Other adverse effects of excessive use of dexamethasone including delayed healing of wounds, retention of salt and water in the body, hypertension, and excessive hairs in different parts of the body such as the face, especially in females (Xue et al., 2014). Glucocorticosteroids stimulate the hepatic glycogen deposition and gluconeogenesis. (Meuleman and Katz, 1985). On the other hand, dexamethasone has a potential for atherogenic effect, given that they cause dyslipidemia and hypertension in humans (Situnayake and Kitas, 1997; Boers et al., 2003) and some animals (Maxwell, 1994). The main cause of these detrimental adverse effects is the overproduction of reactive oxygen species (ROS) by dexamethasone (Bjelaković et al., 2007; Feng and Tang, 2014). In the last few decades, natural products have shown potential therapeutic benefits, including antioxidant (Mohamed and Safwat, 2016; Kandeil et al., 2018 a,b; Kandeil et al., 2019), anti-inflammatory, anti-cancer, antimicrobial, and immunomodulatory effects. (Dattner, 2003; Huffman, 2003). Therefore, they can be used as feed additives for replacement of synthetic compounds in order to treat various diseases and to avoid toxicity, high cost, or excessive residues in meat and livestock products. Among the promising medicinal plants, the fruits of the date palm (Phoenix dactylifera L-Areceae), which is a valuable plant commonly consumed in various parts of the world especially in Arabian countries (Cerezuela et al., 2016). The fruit is called “tamr” in Arabic. The date was mentioned more than any other fruits in Quran (Al-Farsi and Lee, 2008; Marwat et al., 2009). The date fruit has been used for many years in folk medicine for treating cancer and several infectious diseases (Tang et al., 2013). Date palm possesses several highly beneficial properties such as antifungal, antiviral, antioxidant, hepatoprotective and hypolidemic activities (Al-Farsi and Lee, 2008). The dates, either fresh or dried, have high sugar content, low fats and protein contents, as well as minerals, vitamins and antioxidants flavonoids, carotenoids, and polyphenols (Vayalil, 2002; Anjum, 2012). Egyptian date palm fruit extracts were previously analyzed by HPLC. The chromatograms for methanol and aqueous extract of date palm fruit extract revealed higher concentrations of phenolic and flavonoids including Ferulic acid, Chlorogenic acid, Caffeic acid, p-Coumaric acid, Sinapic acid, Quercetin-3-O- glycoside, Apigenin-c-glycoside, and Luteolin- 7- O-glycoside (Hussein et al., 2015). The sugars are responsible for the sweet taste of dates. The dates contain almost half of the amount of sugars in the form of fructose, which is twice as sweet as glucose and can induce a feeling of satiety (Ali et al., 2009). Dates contain high levels of selenium, copper, potassium, and magnesium, moderate concentrations of manganese, iron, phosphorus, and calcium (Khan et al., 2008). Fresh dates contain higher concentrations of vitamins (thiamin, riboflavin, niacin, ascorbic acid, pyridoxine, and vitamin A) as compared to dry dates (Al-Shahib and Marshall, 2003). Enzymes such as phytase, invertase and peroxidase and other isolated chemical constituencies including a-D gulcan, heteroxylan and galactomannans have been isolated from dates, (Mahran et al., 1976). The carotenoids (lutein followed by carotenes) and phenolic compounds (flavonoids, anthocyanins and cinnamic acid) can contribute to varying degrees of antioxidant and antimutagenic activity. The contribution of total phenolics toward antioxidant activity in dates is greater than that of ascorbic acid (Shivashankara et al., 2004). Probiotics and prebiotic feed additives in animals have potential advantages because they are thought to promote intestinal health and may offer a replacement for current intervention strategies that are not considered acceptable for the production systems (Pan and Yu, 2013; Park et al., 2013). Probiotics beneficially affect the host by improving survival and implantation of live microbial dietary supplements in the gastro intestinal flora, by selectively stimulating the growth or activating the catabolism of one or a limited number of health-promoting bacteria in the intestinal tract, and by improving the gastrointestinal tract microbial balance, and improving gastrointestinal barrier function (Nagpal et al., 2012). Probiotics have been used as an alternative to antibiotics in animals and humans; (Siriken et al., 2003). Saccharomyces cerevisiae yeast can be widely used as a natural productivity stimulator added to animals’ feed (Grela and Semeniuk, 2006). Saccharomyces cerevisiae and S. boulardii are clinically proven yeasts being used as a human probiotic and has shown to positively influence host’s health by antimicrobial effect, nutritional effect, inactivation of bacterial toxins, quorum sensing, trophic effects, immuno-modulatory effects, anti-inflammatory effects, cell restitution and maintenance of epithelial barrier integrity (Moslehi-Jenabian et al., 2010). Several previous studies showed that members of Saccharomyces genus can possess anti-bacterial and probiotic properties (Nayak, 2011). Previous studies showed that dexamethasone induces oxidative stress in rat (Feng and Tang, 2014). So increasing the antioxidants levels could be a solution to achieve the reduction of oxidative stress and other side effects resulted from dexamethasone administration. Date palm and/or Saccharomyces cerevisiae probiotics were used in the current study to evaluate the possible protective effects on dexamethasone-induced metabolic changes and liver dysfunction in rabbits. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Materials and methods |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Diet The standard normal rabbit chow was obtained from AL-Dakahlia Factory for feed manufacture, AL-Dakahlia city, Egypt. The ingredients of the diet include clover hay, wheat bran, barley grain, soybean meal, molasses, limestone, sodium chloride, vitamins, and mineral premix. The chemical analysis of the standard normal chow diet is not less than 16.72% crude protein, about 13.07% crude fiber, and digestible energy 24%. Date palm fruit Dry Egyptian date palm fruits were collected from local markets at Beni-Suef city, Egypt. The dates were identified by the Botany Department, Faculty of Sciences, Beni-Suef University, Egypt. Chemicals Dexamethasone (Epidron®) was obtained from Epico Company, Egypt. Probiotic (Levucell SB®) was obtained from Brookside Agra-USA, Company. Triglycerides, cholesterol, HDL-Cholesterol, glucose, reduced glutathione (GSH), malondialdehyde (MDA) and catalase commercial kits were purchased from Biodiagnostic Company for research kits, Egypt. Albumin and alanine aminotransaminase (ALT) commercial kits were purchased from Spinreact Company, Spain. Total proteins and alkaline phosphatase (ALP) kits were purchased from Human commercial kits for research kits, Egypt. The other chemicals used in the experiment are of high grade and have been obtained from Sigma, USA. Animals and grouping A total of 25 healthy male white New Zealand rabbits at the age of 30 days and body weight ranged from 500-600 grams were obtained from the private commercial project for rabbit industry at AL-Dakahlia Governorate, Egypt. The rabbits were housed in individual stainless-steel cages at 23±2 ºC, humidity of 50±10%, with 12 hours light-dark cycle. The rabbits were fed on formulated ration with free access to feed and water throughout the study. Rabbits were acclimated for 2 weeks before starting the experiment. All experimental measures were performed according to the instructions for the care and use of laboratory animals and in accordance with the local Animal Care and Use Committee at Beni-Suef University. Rabbits were equally divided into 5 groups (each includes 5 rabbits) as follows: Group1 (control group): Rabbits were received no treatment. Group 2: Rabbits were injected intramuscularly with dexamethasone (Epidron ®) 2mg/kg body weight (Jeklova et al., 2007) daily, for one month. Group 3: The flesh of date palm was manually separated from the pits and was given to rabbits at a dose of 0.5 g/kg body weight daily orally along with I/M injection of dexamethasone for one month. Group 4: S. cerevisiae probiotic were mixed with ration as 1g/ kg ration (Shehu et al., 2013), concomitant with I/M injection of dexamethasone daily for one month. Group 5: Rabbits were fed ration mixed with Saccharomyces cerevisiae probiotic and date palm flesh concomitant with I/M injection of dexamethasone daily for one month. Blood sampling and tissue preparation 24 hours following the last dose, the blood samples were collected from the marginal ear vein of each rabbit at 10 hours fasting state. Blood samples were left at room temperature for 20 minutes to clot, then centrifuged at 3000 rpm for 15 minutes for serum separation. The sera were monitored for fasting glucose, lipid profile and liver function biomarkers. All rabbits were slaughtered; liver tissues were excised after dissection of the animals then weighed. Liver homogenate was obtained by homogenizing one gram tissue in 10 ml cold phosphate buffer (pH: 7) using homogenizer (Teflon Homogenizer, India) then centrifuged at 20000×g for 15 min at 4ºC. The supernatants were kept at-20ºC until biochemical analysis of GSH, MDA, and catalase. Coefficient weights of liver Body weight was recorded before sacrificing of all animals. After weighing the body and liver, the coefficients of liver to body weight were measured by the ratio of organ weight (mg) to animal body weight (g). Biochemical analysis Glutathione (GSH) and malondialdehyde (MDA) levels, and catalase activity in liver homogenate were measured according to Beutler et al. (1963), Satoh (1978) and Aebi (1984) respectively. The serum was used for the estimation of total proteins by following the Biuret method (Henry, 1964) and albumin by following the bromocresol green method (Doumas and Biggs, 1972), Alanine transaminase (ALT), (Reitman and Frankel, 1957), alkaline phosphatase (ALP) (Tietz et al., 1983), triglycerides (Fassati and Prencipe 1982), cholesterol (Allain et al., 1974), HDL-Cholesterol (Kostener, 1977) and glucose (Trinder, 1969). Serum globulins levels were calculated by subtraction of albumin from total serum proteins, A/G ratio was calculated by dividing albumin concentration on globulins concentration (Noverraz, 1953). VLDL-cholesterol was calculated as TG/5 while LDL-cholesterol was calculated by the formula [LDL-c = total cholesterol-(HDL-c+VLDL-c)] according to Friedewald (1972) and Fruchart (1982). Risk ratio was calculated by the equation (LDL-c/HDL-c) described by Lemieux et al. (2001). All chemical reactions were measured by using Hitachi spectrophotometry, Model U – 2000 (Hitachi L td. Tokyo, Japan). Statistical analysis Preliminary statistical analysis was carried out using GraphPad InStat software (version 3, ISS-Rome, Italy). Unless differentially specified, groups of data were compared with unpaired T. test and one-way analysis of variant (ANOVA) followed by Tukey-Kramer (TK) multiple Comparison post-test. The data as clearly indicated are reported in tables and figures as mean ± standard error (SEM).SE. Values of P ≤ 0.05 were regarded as significant. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Results |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
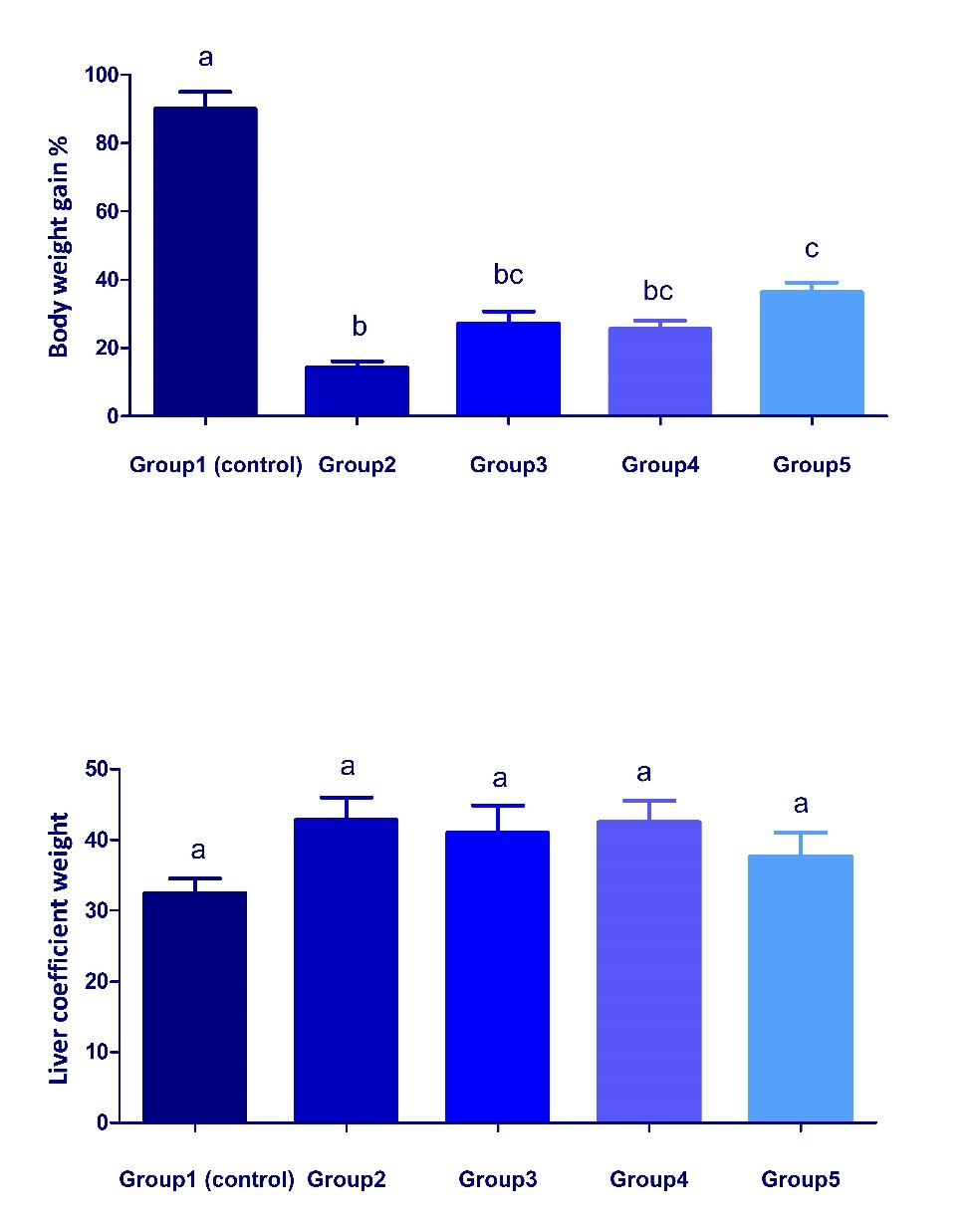
Changes in body weight gain % and liver coefficient in different treated groups Body weight gain % significantly decreased (P < 0.05) in group 2 compared with the control group (Fig. 1). To some extent, the co-treatment with date palm fruit and/or probiotics could modulate such decrease of body weight gain % but still lower than normal levels. The combination of date palm with S. cerevisiae probiotic exerted a better effect than the use of each alone. Although, an increase in the liver coefficient was observed in all treated groups compared with the control group but it was non-significant.
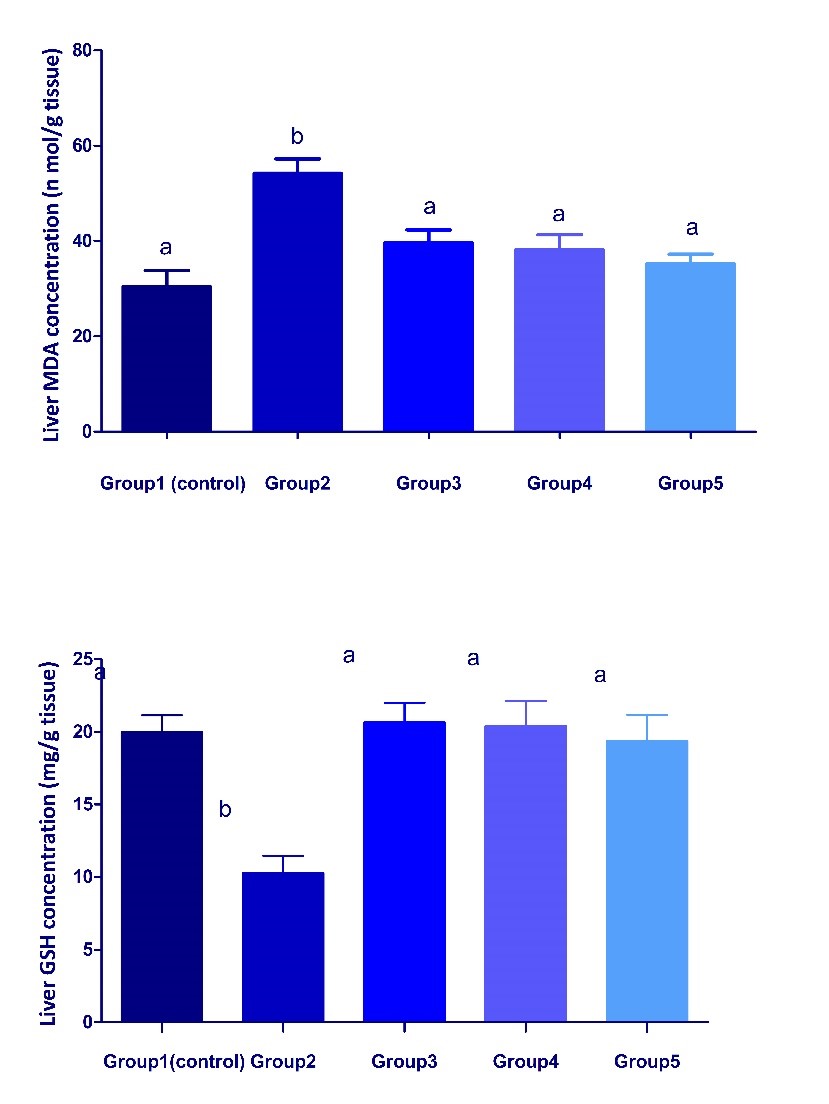
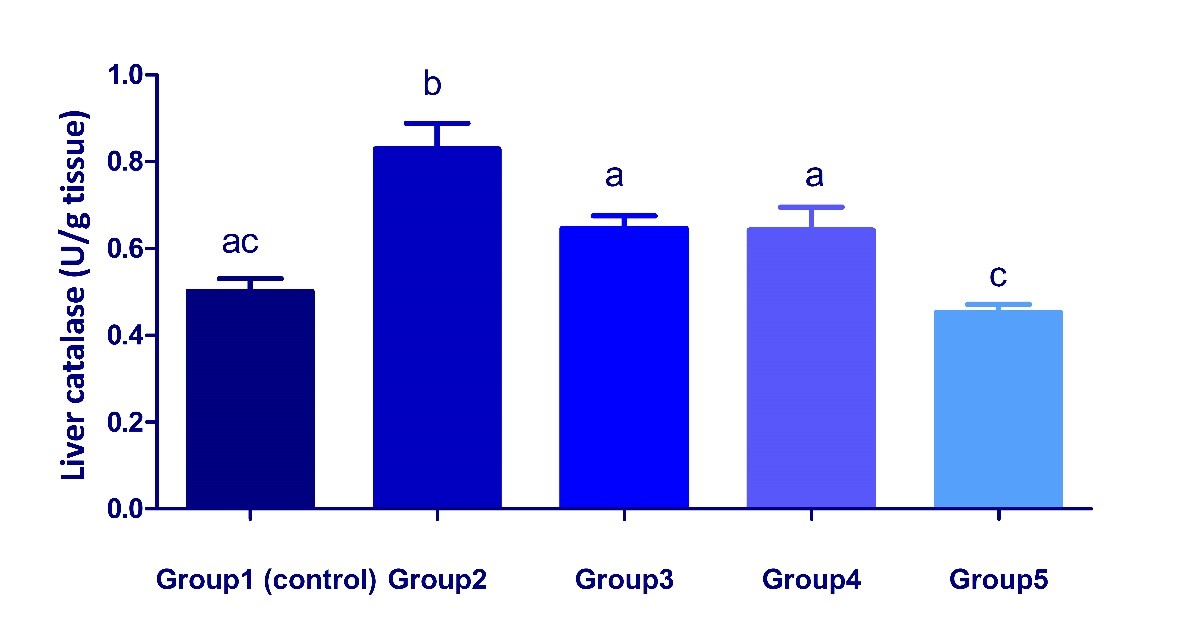
Fig. 1. Changes in body weight gain and liver coefficient in different treated groups. Values are represented as mean ± SEM. (n = 5). Bars show the mean and SEM. Different superscript letters (a, b,c) mean a significant difference at (P <0.05) among different groups in the same column. Changes in the antioxidant status in liver of different treated groups Dexamethasone administration significantly (P < 0.05) increased MDA concentration and catalase activity but significantly decreased GSH concentration in liver of group 2 compared with the control group (Fig. 2, 3). These parameters significantly attenuated in groups 3, 4 and 5, when compared with group 2. The results reflect an ameliorative effect of both date palm and/or S. cerevisiae probiotic on dexamethasone- induced oxidative stress.
Fig. 2. Changes in MDA and GSH concentrations in liver of different treated groups. Values are represented as mean ± SEM. (n = 5). Bars show the mean and SEM. Different superscript letters (a, b) mean a significant difference at (P <0.05) among different groups in the same column.
Fig. 3. Changes in catalase activity in liver of different treated groups. Values are represented as mean ± SEM. (n = 5). Bars show the mean and SEM. Different superscript letters (a, b,c) mean a significant difference at (P <0.05) among different groups in the same column. Changes in the serum liver function biomarkers in different treated groups The data in a Table 1, showed significant decreases in total proteins, albumin concentrations, ALT, and ALP activities in group 2 when compared with the control. On the other hand, the co-treatment with additives (date palm and /or S. cerevisiae) parallel with dexamethasone administration showed an ameliorative effect as the values significantly returned to control. Globulins concentration and A/G ratio showed non-significant variations among treated groups (Table 1). Table 1. Changes in serum liver function tests in different treated groups.
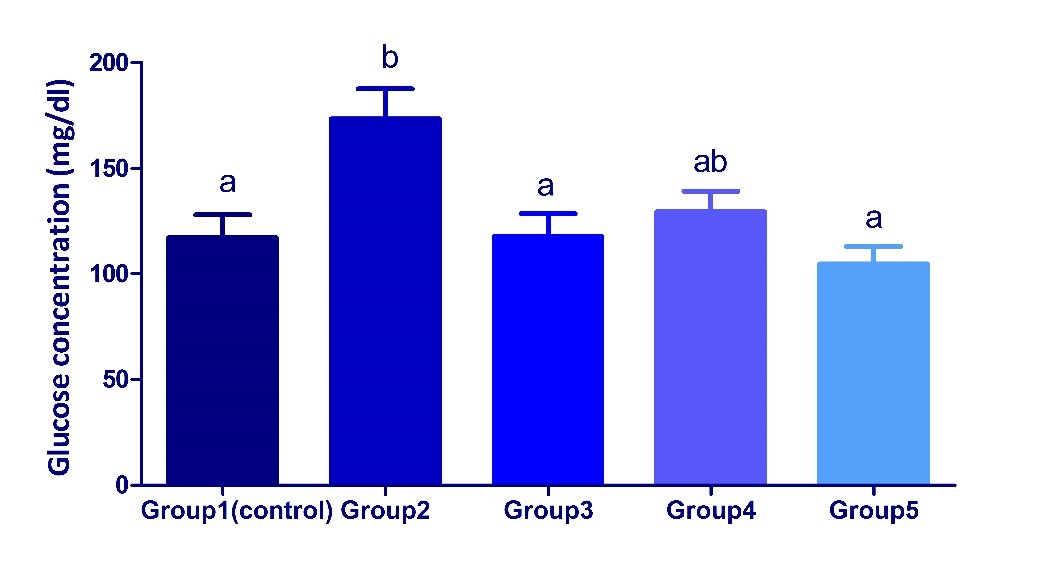
Values are represented as mean ± SEM. (n = 5). Different superscript letters (a, b) mean a significant difference at (P <0.05) among different groups in the same column. A/G Ratio: Albumin/ Globulins Ratio, ALT: Alanine aminotransferase, ALP: Alkaline phosphatase Changes in serum glucose concentrations in different treated groups There were significant increases in serum concentrations of glucose in Dexamethasone treated group in comparison with the control group, while the co-treatment with additives (date palm and /or S. cerevisiae) parallel with dexamethasone administration showed an ameliorative impact as the concentrations of glucose returned nearly to control (Fig. 4).
Fig. 4. Changes in serum glucose concentrations in different treated groups. Values are represented as mean ± SEM. (n = 5). Bars show the mean and SEM. Different superscript letters (a, b) mean a significant difference at (P <0.05) among different groups in the same column. Changes in serum lipid profile and risk ratio in different treated groups The levels of TG, VLDL, TC and LDL/HDL risk ratio showed significant (p<0.05) increases in serum of dexamethasone treated group as compared with control. In contrast, HDL levels showed an insignificant decrease, while LDL levels showed an insignificant increase in serum of dexamethasone treated group when compared with the control one (Table 2). On the other hand, the co-treatment with additives (date palm and /or S. cerevisiae) parallel with dexamethasone administration showed an ameliorative effect by the restoration of the values to the near normalcy. However, the degree of improvement was markedly higher in groups 4 and 5, than group 2 reflecting the more hypolipidemic effect of S. cerevisiae probiotics over date palm fruits. Table 2. Changes of serum lipid profiles and Risk ratio in different groups.
Values are represented as mean ± SEM. (n = 5). Different superscript letters (a, b,c) mean a significant difference at (P <0.05) among different groups in the same column. TG: Triacylglycerols, TC: Total cholesterol, VLDL: Very Low Density lipoprotein, LDL: Low Density lipoprotein, HDL: High Density lipoprotein. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Discussion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Rabbits have been used in the present study as an experimental model for mammals (Jeklova et al., 2008; Randhawa, 2008). Also, the present study was conducted by using prolonged administration of dexamethasone sodium phosphate to induce unwanted side effects on the organ system and metabolic reactions. In the current study, body weight gain % significantly decreased while liver coefficient slightly increased in dexamethasone group compared with the control group (Fig. 1). To some extent, date palm and/or S. cerevisiae probiotics could modulate such decrease in body weight gain values to near normal levels (Fig. 1). It is remarkable that the treatment with both dates and probiotics together with dexamethasone in group 5 had a better effect than the use of either treatment alone. Our results come in agreement with Bennett and Brown (2003), Amar et al. (2013) and Debangadas et al. (2017) who reported that dexamethasone markedly decreased the body weight and protein anabolism, and with Klymenko and Kozyrieva (2002); Jeklova et al. (2008) and Al-Sa'aidi et al. (2012) who recorded a significant increase in the liver and kidney weights of rabbits treated with dexamethasone. The reduction in the body weight gain of rabbits detected in this study was attributed to the increased leptin production (Murakami et al., 1995; Miell et al., 1996; Reul et al., 1997) and the excessive lipolysis (Rebuffé-Scrive et al., 1988) induced by administration of high catabolic dose of dexamethasone (2mg/kg). The slight increment in liver weight coefficient could be attributed to a rapid mobilization of depot fat with the uptake of free FA to the liver and its deposition in the form of triglycerides in response to glucocorticoids (Mahendran and Shyamala Devi, 2001; Schwarz et al., 2003). Date palm and probiotic treatment lead to the increment in nutritional value and antioxidant status of animals that in turn improves their growth performance, production, and reproduction (Wu et al., 2013). Yeast-derived polysaccharides such as beta-glucans and mannans have been considered as potential growth and health promoters for modern livestock and poultry production (Shashidhara and Devegowda, 2003; Volman et al., 2008) and growth promoter for intestinal microflora (Spring et al., 2000). These results also were confirmed by the findings of several researches as Zhang et al. (2005), Paryad and Mahmoudi (2008). The improvement in the body weight of birds fed S. cerevisiae could be attributed to its direct nutritional effect (Patterson and Burkholder, 2003) and its fermentation products. S. cerevisiae is a naturally rich source of proteins, minerals and B-complex vitamins (Huff et al., 2007). The generation of ROS and oxidative injury is thought to play a significant role in many of the observed adverse effects of dexamethasone (Bjelaković et al., 2007; Feng and Tang, 2014). Liver is a major target organ for GCs metabolic reactions and is considered the main organ generating ROS in different pathological conditions (Socha et al., 1992). Our data showed a significant increase in MDA concentration and catalase activity and a significant decrease of GSH concentration in liver homogenates obtained from dexamethasone treated rats compared with the control (Fig. 2, 3). In accordance with our findings, Bjelaković et al. (2007) found that dexamethasone treatment leads to oxidative damage that affects the biological structures. Grubinko et al. (2001) recorded that cortisol and its derivatives induce oxidative reactions such as damaging DNA, activating lipid peroxidation, and decreasing the antioxidant defense. It is worth mentioning that, the increased activity of catalase in liver might be due to a compensatory response to oxidative stress. The obtained results showed an ameliorative effect of both date palm and S. cerevisiae on oxidative stress caused by dexamethasone where the values returned to normal control levels (Figs. 2, 3). Such effect may be attributed to the antioxidant effect of p-coumaric acid, ferulic acid, flavonoids, and procyanidins in date palm. Similar results were reported by Vayalil (2002); Orabi and Shawky (2014) and El-Far et al. (2016). Saccharomyces cerevisiae has been reported with strong antioxidant activity, reducing power, nitric oxide and hydroxyl radical scavenging activity, metal ion chelating activity (Hassan, 2011; Fakruddin et al., 2017). Tests of the biosynthetic function of the liver include serum total proteins, albumin and globulins, which are synthesized in the liver and transported into the circulation (Doweiko and Nompleggi, 1991). Hepatic injury can be evaluated by determining the concentrations of some serum specific indicators, which are associated with hepatocellular necrosis (ALT and AST), cholestasis (ALP) and altered excretion (Bilirubin) (Wolf, 1999). Results in a Table 1, showed a significant decrease in both serum total proteins and albuminconcentration and a significant increase in both serum ALT and ALP activities in dexamethasone treated group in comparison with the control group. Serum globulins concentration and albumin/ globulins ratio were non- significantly changed in all treated groups. Results from the present study are in accordance with the findings of Abd Elazem and Abo-Kora (2015). Moreover, some hepatic lesions were reported after dexamethasone administration including cytoplasmic fatty vacuolation and necrosis of the hepatocytes (Amar et al., 2013) and obstruction of bile flow that results in the development of oxidant injury, liver fibrosis, biliary cirrhosis and portal hypertension (Eken et al., 2006). These present findings indicated hepatocytes damage induced by dexamethasone that in turn alters the protein synthetic function and membrane permeability as well as leakage of intracellular ALT and ALP enzymes from the damaged liver cells. Additionally, increased ALP has been linked with lack of bile flow and increased serum cholesterol level (Oyetayo et al., 2003), which was detected in the present study. The co-treatment with additives (dates palm and /or S. cerevisiae) parallel with dexamethasoneadministration showed an ameliorative effect as the values nearly return to the control levels (Table 1). In accordance with the current results, Al- Qarawi et al. (2004) found that aqueous extracts of flesh and pits of dates caused a significant reduction in liver enzymes and elevation in serum albumin in CCl4-induced hepatotoxicity rat model. Usama et al. (2009) reported the same findings in CCl4 hepatotoxic rabbits treated with Siwa date palm extract. Date palm has been known for a lot of beneficial properties such as antioxidant and hepatoprotective activities (Wan Ismail and Mohd Radzi, 2013). This hepatoprotective effect for palm date extracts could be attributed to its antioxidant contents. Mansouri et al. (2005) analyzed the phenolic profile of seven Algerian varieties of date and detected the existence of high concentrations of p-coumaric, ferulic and sinapic acids, and some cinnamic acid derivatives. Also, Hong et al. (2006) indicated the presence of quercetin and luteolin in mature Deglet Noor dates. The ameliorative effect of probiotics on liver functions was previously indicated in several reports. In this context, S. cerevisiae-supplemented diets could markedly increase serum total proteins and albumin concentrations in Lambs (Wójcik, 2010) and in ewes under low nutrition condition (Aly et al. 2013). S. cerevisiae showed similar activities of ALT and ALP in both treated and control group mice (Fakruddin et al., 2017). Lai et al. (2009) and Sener et al. (2006) all reported that a fermentative product and also β-glucan that were derived from S. cerevisiae recovered the liver damage in rats by the restoration of AST and ALT activities toward the normal. In contrast, the addition of S. cerevisiae significantly increased the activities of serum ALT and ALP (Mannaa et al., 2005). Figure 4, showed a significant increase in glucose level in dexamethasone treated group compared with the control group. In the present study, the metabolic disorders caused by dexamethasone was in agreement with Pagana and Pagana (2010) and Amar et al. (2013). Glucocorticoids in excess directly inhibit the insulin signaling pathway and the translocation of glucose transporter GLUT4 away from the plasma membrane into the cell, thereby decreasing glucose uptake and utilization in muscles and inhibits glycogen synthesis (Weinstein et al., 1998) with subsequent higher plasma glucose levels (Mazziotti et al., 2011). As a consequence, glucagon secretion, lipolysis, proteolysis and hepatic glucose production increased (Das et al., 2017). Glucocorticoids induce hepatic insulin resistance by stimulating hepatic gluconeogenesis via up-regulation of gene transcription of phosphoenolpyruvate carboxykinase, glucose-6- phosphatase and tyrosine aminotransferase (Hanson and Reshef, 1997; Reichardt and Schutz, 1998). The co-treatment with additives (Date palm and /or S. cerevisiae) parallel with dexamethasone administration showed an ameliorative impact as the concentrations of glucose return nearly to control (Fig. 4). This result was in agreement with Zangiabadi et al. (2011) and Hussein et al. (2015) who proved that date fruit extracts decreased the fasting blood glucose levels and prevent diabetes risks in diabetic rats. Similarly, the date seed extract also decreased blood glucose in male diabetic rats (Mokhtari et al., 2008). On the other hand, treatment with S. cerevisiae reduced blood glucose and triglycerides in agreement with Silva et al. (2015) and Andrade et al. (2016). The glycemic control of S. cerevisiae could be mediated by different mechanisms. Beta-glucan fibers, derived from the yeast cell wall, probably form a gelatinous layer in the intestinal lumen and delay the absorption of carbohydrates and lipids by enterocytes (Choi et al., 2010; Silva et al., 2015). Another possible mechanism is mediated by the activation of PI3K/Akt signal pathway by beta-glucans to reduce blood glucose level (Chen and Seviour, 2007). The altered adipose tissue metabolism by glucocorticoids may contribute to insulin resistance and altered insulin secretion associated with a higher risk of type 2 diabetes (Fardet et al., 2013). Dyslipidemia is considered a risk factor for cardiovascular disorders. The accumulation of triglycerides in the myocardium may be one of the underlying mechanisms for the development of cardiac steatosis (McGavock et al., 2007), and hypercoagulation (Trementino et al., 2010). Moreover, the direct effects of glucocorticoids on the heart are due to the expression of receptors in cardiomyocytes (Ren et al., 2012). The mechanism underlying glucocorticoid-induced dyslipidemia involves the induction of lipolysis in adipose tissue through the activation of hormone-sensitive lipase and an increased catecholamine response (Slavin et al., 1994), as well as the increased VLDL production and the decreased fatty acid B-oxidation (Dolinsky et al., 2004). The excessive lipolysis with the delivery of free FA to the liver will drive the hepatic de novo lipogenesis (Schwarz et al., 2003). The results in a Table 2, showed the lipid profile in which significant increases in triglycerides, total cholesterol, and VLDL levels were detected. An insignificant increase in LDL levels and an insignificant decrease in HDL levels were also observed in dexamethasone treated group compared with the control one. Results from this study, come in agreement with Mahendran and Shyamala Devi (2001), Amar et al., (2013) and Arab Dolatabadi and Mahboubi (2015). In the current study, the increases in triglyceride, total cholesterol, VLDL, and LDL circulating levels and a decrease in HDL cholesterol levels are the major metabolic disturbances resulted from excess GCs. Hypertriglyceridemia may result from enhanced hepatic lipogenesis via upregulation of both acetyl-CoA carboxylase and fatty acid synthase (Zhao et al., 2010) with increased hepatic synthesis of VLDL (Dolinsky et al., 2004) and reduced peripheral clearance due to reduced activity of lipoprotein lipase after dexamethasone administration (Mahendran and Shyamala Devi (2001). The hypercholesterolemic effect of dexamethasone might be attributed to the activation of cholesterol biosynthesis via activation of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase (Kobashigawa and Kasiske, 1997), and the decreased activity of lecithin: cholesterol acyltransferase, an enzyme which plays a key role in incorporating the free cholesterol into HDL which is then catabolized in liver (Mahendran and Shyamala Devi, 2001). Furthermore, the down‐regulation of LDL receptor, and lipoprotein lipase activities are the main reasons for the elevation of total cholesterol, LDL, and VLDL levels (Kobashigawa and Kasiske, 1997). A significant increase in the serum LDL/HDL cholesterol mean ratio was observed in dexamethasone treated rats (Table 2) indicating the high risk of cardiovascular disease (Karthikeyan et al., 2007). On the other hand, the dexamethasone-induced hyperlipidemia was minimized by co-treatment with date palm and/or S. cerevisiae as indicated by restoration of the values to the near normalcy. However, the results revealed the more hypolipidemic effect of S. cerevisiae probiotics over date palm fruits (Table 2). The obtained results come in agreement with Hassan et al. (2010) who found that date extracts induced a significant increase in HDL and a significant decrease in total cholesterol, LDL, TG and LDL/HDL ratio in diet-induced hypercholesterolemic rabbits. Kumar et al. (2012) reported that the levels of cholesterol and triglycerides were decreased in yeast fed piglets as compared to control. Among the beneficial properties of date palm is antihyperlidemic activity (Wan Ismail and Mohd Radzi, 2013). The possible mechanism of action for the hypolipidemic effect observed in date palm is the presence of antioxidants like polyphones and flavonoids as well as the presence of dietary fiber in date palm which may reduce the absorption of dietary fats leading to improvement in lipid profile (Musa, 2018). Numerous studies have proved that a high level of HDL is associated with a lower incidence of cardiovascular disease (Assmann and Nofer, 2003). Moreover, Rock et al. (2009) also reported that after 4 weeks of date palm consumption, the VLDL reduced. It is well known that dietary fiber is able to decrease LDL concentration by interfering with cholesterol absorption and enterohepatic bile circulation and increasing LDL receptor activity. The finding of these study suggested that date palm could have a protective effect against hyperlipidemia through the improvement of lipid profile (Abuelgassim, 2010). A significant lipid-lowering effect subsequent to the co-treatment with S. cerevisiae was previously reported by Seyidoglu et al. (2013) in male rabbits, Ahmadi (2011) and Oie and Liong (2010) in chickens and Kumar et al. (2012) in piglets. It has been hypothesized that the principal mechanism for the lipid-lowering action of probiotics was resulted from decreased hepatic de novo lipogenesis and decreased VLDL production (Trautwein et al., 1995). This hypothesis was supported by Delzenne et al. (1999), Gallaher (2000) and El Mahmoudy et al. (2014) who found an improving effect of the dietary supplementation with mannan-oligosaccharide (naturally present in the cell walls of the yeast S. cerevisiae) on the lipogram in hyperlipidemic rats. The hypocholesterolemic effect of probiotics may result from the increased viscosity of intestinal contents caused by mannan feeding that was potentially associated with the lowering of cholesterol absorption (Gallaher et al., 2000). Probiotics have the ability to deconjugate with bile acids, enzymatically increasing their rate of excretion and the use of cholesterol to synthesize new bile lead to the reduction of serum cholesterol level (Lye et al., 2009). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conclusion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The present study revealed the potential ameliorative effect of both feed additives (date palm fruits and S. cerevisiae probiotics) against dexamethasone-induced metabolic alterations in rabbits. The main findings of this study were related to the improved antioxidant status, liver function, glycemic control, lipid profile and cardiovascular protection in rabbits subjected to dexamethasone administration. Furthermore, it suggested the potential hypolipidemic effect of S. cerevisiae probiotic over the date palm in rabbits. Therefore, the combination of both date palm fruits and S. cerevisiae probiotics could potentially be considered of great interest as feed additives. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Acknowledgments |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The authors thank the staff members of the Biochemistry Departments at Beni-Suef University and Animal Health Research Institute, for their help and advice. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conflict of Interests |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
No potential conflicts of interest were disclosed. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
References |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abd Elazem, M.A., Abo-Kora, S.Y., 2015. Adverse effects of Diclofenac Potassium and Dexamethason on some hematobiochemical and immunological parameters in Egyptian goat bucks. J. Am. Sci. 11, 92-99. Abuelgassim, A.O., 2010. Effects of Flax seed and date palm leaves extract on serum concentration of glucose and lipids in alloxan diabetic rats. Journal of Biological Science 13, 1141-1145. Aebi, H., 1984. Catalase in vitro. Methods Enzymol. 105, 121-126.
Ahmadi, F., 2011. The effects of Saccharomyces cerevisiae (Thepax) on performance, blood parameters and relative weight of lymphoid organs of broiler chicks. Global Veterinaria 6, 471-475. Al-Farsi, M.A., Lee, C.Y., 2008. Nutritional and functional properties of dates: A review. Critical Review in Food Science and Nutrition 48, 877–884. Ali, A., Al-Kindi, Y.S., Al-Said, F., 2009. Chemical composition and glycemic index of 3 varieties of Omani dates. International Journal of Food Science and Nutrition 60, 51–62. Allain, C. C., Poon, L.S., Chan, C.S., Richmond, W., Fu, P.C. 1974. Enzymatic determination of total serum cholesterol. Clin. Chem. 20, 470-475.
Al-Sa'aidi, J.A.A., Dawood, KH. A., Latif, A.D., 2012. Immunomodulatory effect of Nigella sativa seed extract in male rabbits treated with dexamethasone. Iraqi Journal of Veterinary Sciences 26, 141-149. Al-Shahib,W., Marshall, R.J., 2003. The fruit of date palm: Its possible uses as best food for the future. International Journal of Foods Science and Nutrition 54, 247–259. Al- Qarawi, A.A., Mousa, H.M., Ali, B.H., Abdel-Rehman, H., El Moughy, S.A., 2004. Protective effect of extracts from Date palm on Carbon Tetra chloride- induced hepatotoxicity in Rats. Int. J. Appl. Res. Vet. Med. 2, 176-180. Aly, Sahar, A., Mahmoud, Mona, A., Naglaa, 2013. Effect of probiotic supplementation on DNA fragmentation, some haematological, biochemical and antioxidant parameters in sheep. Egypt. J. Basic Appl. Physiol. 12, 319-37. Amar, M.I., Adam Shama, I.Y., Enaia, A.A., Hind, A.E.O., Hager, A.M., 2013. Effects of Various Levels of Oral Doses Dexamethasone (Al-nagma) Abused as Cosmetic by Sudanese Women on Wistar Rats. Journal of Medical Sciences 13, 432-438. Anjum, F.M., Bukhat, S.I., El-Ghorab, A.H., Khan, M.I., Nadeem, M., Hussein, S., Arshad, M.S., 2012. Phytochemical characteristics of date palm (Phoenix dactylifera) fruit extracts. Pak. J. Food Sci. 22, 117-127. Andrade, E.F., Lima, A.R., Nunes, I.E., Orlando, D.R., Gondim, P.N., Zangeronimo, M.G., Pereira, L.J., 2016. Exercise and Beta-Glucan Consumption (Saccharomyces cerevisiae) Improve the Metabolic Profile and Reduce the Atherogenic Index in Type 2 Diabetic Rats (HFD/STZ). Nutrients 8, 792. doi:10.3390/nu8120792 Andrews, A.H., Laven, R., Maisey, I., 1991. Treatment and control of an outbreak of fat cow syndrome in a large dairy herd. Vet. Rec. 129, 216–219. Arab Dolatabadi, A., Mahboubi, M., 2015. A study of the influence of dexamethasone on lipid profile and enzyme lactate dehydrogenase. Journal of Medicine and Life 8, 72-76. Assmann, G., Nofer, J.R., 2003. Athero protective effects of High density lipoproteins. Journal of Medical Sciences 54, 321-341 Bennett, P.N., Brown, M., 2003. Clinical Pharmacology. 9th. (eds.). Churchill Livingstone, pp: 663- 675. Beutler, E., Duran, O., Kelly, MB. J., 1963. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 61, 882-888.
Bjelaković, G., Beninati , S., Pavlović, D., Kocić, G., Jevtović, T., Kamenov, B., Saranac, L. J., Bjelaković, B., Stojanović, I., Basić, J., 2007. Glucocorticoids and oxidative stress. J. Basic Clin. Physiol. Pharmacol. 18, 115-127. Boers, M., Nurmohamed, M.T., Doelman, C.J., Lard, L.R., Verhoeven, A.C., Voskuyl, A.E., Huizinga, T.W., Van de stadt, R.J., Dijkmans, B.A., Van der Linden, S., 2003. Influence of glucocorticoid and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann. Rheum. Dis. 62, 842-845. Cerezuela, R., Guardiola, F.A., Cuesta, A., Esteban, M.A., 2016. Enrichment of gilthead seabream (Sparus aurata L.) diet with palm fruit extracts and probiotics: Effects on skin mucosal immunity, Fish. Shellfish Immunol, 49, 100e109. Chen, J., Seviour, R., 2007. Medicinal importance of fungal beta-(1-->3), (1-->6)-glucans. Mycol. Res. 111(Pt 6),635-652.
Choi, J.S., Kim, H., Jung, M.H., Hong, S., Song, J., 2010. Consumption of barley beta-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res, 54, 1004–1013. Czock, D., Keller, F., Rasche, F.M., Haussler, U., 2005. Pharmacokinetics and pharmacodynamics of systematically administered glucocorticoids. Clin. Pharmacokinet. 44, 61–98. Dattner A.M., 2003. From medical herbalism to phytotherapy in dermatology: back to the future, Dermatol. Ther. 16, 106–113. Das, D., Chakraborty, J., Dash, S., 2017. Bioequvalance study of antidiabetic activity between two marketed formulations of metformin on glucocorticoid-induced hyperglycemia in rabbit. Int. J. Curr. Pharm. Res. 9, 47-50. Delzenne, N.M., Kok, N.N., 1999. Biochemical basis of oligofructose-induced hypolipidemia in animal models. J. Nutr. 129, 1467S-1470S. Dolinsky, V.W., Douglas, D.N., Lehner, R, Vance, D.E., 2004. Regulation of the enzymes of hepatic microsomal triacylglycerol lipolysis and re-esterification by the glucocorticoid dexamethasone. Biochem J. 378 (Pt 3), 967–74. Doumas, B.T., Watson, W.A., Biggs, H.G., 1971. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chem. Acta, 31, 87-96. Doweiko, J.P., Nompleggi, D.J., 1991. Reviews: Role of Albumin in Human Physiology and Pathophysiology. Journal of Parenteral and Enteral Nutrition 15, 207–211.
Eken, H., Ozturk, H., Ozturk, H., Buyukbayram, H., 2006. Dose-related effects of dexamethasone on liver damage due to bile duct ligation in rats. World J. Gastroenterol, 12, 5379-5383. El-Far, A.H., Ahmed, H.A., Shaheen, H.M., 2016. Dietary Supplementation of Phoenix dactylifera Seeds Enhances Performance, Immune Response, and Antioxidant Status in Broilers. Oxid. Med. Cell Longy. Article ID 5454963, 9 pages. El-Gazzar, U.B., El-Far, A.H., Abdel maksoud, H.A., 2009. The Ameliorative Effect of Phoenix Dactylifera Extract on CCl4 Hepatotoxicity in New Zealand Rabbits. Journal of Applied Sciences Research 5, 1082-1087. El-Mahmoudy, A.M., Abdel-Fattah, F.A., Abd El-Mageid, A.D., Gheith, I.M., 2014. Effect of the growth promotant mannan-oligosaccharide on the lipogram and organ function profile in hyperlipidemic albino rats. AJPCT 2, 334-347. Fakruddin, Md., Nur Hossain, Md., Ahmed, M.M., 2017. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complementary and Alternative Medicine 17, 64-74. Fardet, L., Antuna-Puente, B., Vatier, C., Cervera, P., Touati, A., Simon, T., Capeau, J., Feve, B., Bastard, J.P., 2013. Adipokine profile in glucocorticoid-treated patients: baseline plasma leptin level predicts occurrence of lipodystrophy. Clin. Endocrinol. (Oxf) 78, 43–51. Fassati, P., Prencipe, L., 1982. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 28, 2077-2080.
Feng, Y.L., Tang, X.L., 2014. Effect of glucocorticoid-induced oxidative stress on the expression of Cbfa1. Chem. Biol. Interact. 207, 26-31. Friedewald, W.T., Levy, R.I., Fredrickson, D.S., 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502. Fruchart, G.G., 1982. LDL-cholesterol determination after separation of low density lipoprotein. Rev. Fr. Des Laboratories 103, 117. Gallaher, C.M., Munion, J., Hesslink, R., Wise, J., Gallaher, D.D., 2000. Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J. Nutrit. 130, 2753-2759. Grela, E.R., Semeniuk, V., 2006. Consequences of the withdrawal of antibiotic growth promoters from animal feeding. Medycyna Wet 62, 502-507. Grubinko, V.V., Leus, Yu. V., Arsan, O.M., 2001. Lipid peroxidation and antioxidant defense in fish. Gidrobiol. .Zh., 37, 64-78. Hanson, R.W., Reshef, L., 1997. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu. Rev. Biochem. 66, 581–611. Hassan, N.S., Amom, Z.H., Nor, A.I., Arapoc, J.D., Azlan, A., 2010. The Role of Dates (Phoenix dactylifera) Aqueous Extract in Improving the Plasma Lipid Profiles of Diet-Induced Hypercholesterolemic Rabbits. Res. J. Biol. Sci, 5, 632-637. Hassan, H.M.M., 2011. Antioxidant and immunostimulating activities of Yeast (Saccharomyces cerevisiae) autolysates. World Appl. Sci. J. 15, 1110–1119. Henry, R.J., 1964. Clinical Chemistry. Harper and Row Publishers, New York. pp. 181. Hong, Y.J., Tomas-Barberan, F.A., Kader, A.A., Mitchell, A.E., 2006. The flavonoid glycosides and procyanidin composition of Deglet Noor dates (Phoenix dactylifera). J. Agric. Food Chem, 54, 2405-2411. Huff, G.R., Huff, W.E., Rath, N.C., Solis de los Santos, F., Farnell, M.B., Donoghue, A.M., 2007. Influence of hen age on the response of turkey poults to cold stress, Escherichia coli challenge, and treatment with a yeast extract antibiotic alternative. Poult. Sci, 86, 636–642. Huffman, M.A., 2003. Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants. Proc. Nutr. Soc. 62, 371–381. Hussein, A.M., El-Mousalamy, A.M.D., Hussein, S.A.M., Mahmoud, S.A., 2015. Effects of palm dates (Phoenix dactylifera L) extracts on hepatic dysfunctions in type 2 diabetic rat model. World Journal of Pharmacy and Pharmaceutical Science 4, 62-79. Jeklova, E., Leva, L., Jaglic, Z., Faldyna, M., 2008. Dexamethasone- induced immunosuppression: a rabbit model. Veterinary Immunology and Immunopathology. 122, 231- 240. Kandeil, M.A.M., Hassanin, K.M.A., Mohammed, E.T., Safwat, G.M., Mohamed, D.S., 2018a. Wheat germ and vitamin E decrease BAX/BCL-2 ratio in rat kidney treated with gentamicin. Beni-Suef University Journal of Basic and Applied Sciences 7, 257-262. Kandeil, M.A.M., Hassanin, K.M.A., Mohammed, E.T., Safwat, G.M., Mohamed, D.S., 2018b. Pumpkin and Vitamin E as Potent Modulators of Apoptosis in Gentamicin-induced Rat Nephrotoxicity. Asian Journal of Biochemistry 13, 1-8. Kandeil, M.A., Mohammed, E.T., Hashem, K.S., Aleya, L., Abdel Daim, M.M., 2019. Moringa seed extract alleviates titanium oxide nanoparticles (TiO2-NPs)-induced cerebral Q1 oxidative damage, and increases cerebral mitochondrial viability. Environmental Science and Pollution Research. In Press. https://doi.org/10.1007/s11356-019-05514-2. Karthikeyan, K., Bai, B.R.S., Devaraj, S.N., 2007. Efficacy of grape seed proanthocyanidins on serum and heart tissue lipids in rats subjected to isoproterenol-induced myocardial injury. Vascul. Pharmacol. 47, 295–301. Khan, M.N., Sarwar, A., Wahab, M.F., Haleem, R., 2008. Physio-chemical characterization of date varieties using multivariate analysis. Journal of Food Agriculture 88, 1051–1059. Klymenko, M., Kozyrieva, H., 2002. Effect of Dexamethasone on mast cell reaction in inflammation. Fiziol. Zh. 48, 29- 33. Kobashigawa, J. A., Kasiske, B.L., 1997. Hyperlipidemia in solid organ transplantation. Transplantation. 63, 331–338. Kostener, C.M., 1977. Enzymatic determination of cholesterol high density lipoprotein fraction prepared by polyanion precipitation. J. Clin. Chem. 22, 695. Kumar, S., Verma, A.K., Mondal, S.K., Gupta, M., Patil, A.K., Jangir, B.L., 2012. Effect of live Saccharomyces cerevisiae feeding on serum biochemistry in early weaned cross bred piglets Vet. World 5, 663-666. Lai, J.T., Hsieh, W.T., Fang, H.L., Lin, W.C., 2009. The protective effects of a fermented substance from Saccharomyces cerevisiae on carbon tetrachloride-induced liver damage in rats. Clin. Nutr. 28, 338–345. Lemieux, I., Lamarche, B., Couillard, C., Pascot, A., Cantin, B., Bergeron, J., Dagenais, G.R., Després, J.P., 2001. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern. Med. 161, 2685-2692. Liu, D., Ahmet, A., Ward, L., Krishnamoorthy, P., Mandelcorn, E.D., Leigh, R., Brown, J.P., Cohen, A., Kim, H., 2013. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma and Clinical Immunology 9, 30. Lye, H.S., Kuan, C.Y., Ewe, J.A., Fung, W.Y., Liong, M.T., 2009. The improvement of hypertension by probiotics: Effects on cholesterol, diabetes, renin and phytoestrogens. International Journal of Molecular Science 10, 3755-3775. Mahendran, P., Devi, C.S., 2001. Effect of Garcinia cambogia extract on lipids and lipoprotein composition in dexamethasone administered rats. Indian Journal of Physiology and Pharmacology 45, 345-350 Mahran, G.H., Abdel-wahab, S.M., Attia, A.M., 1976. A phytochemical study of date pollen. Plant Medicine 29, 171-175. Mannaa, F., Ahmed, H.H., Estefan, S.F., Sharafa, H.A., Eskander, E.F., 2005. Saccharomyces cerevisiae intervention for relieving flutamide-induced hepatotoxicity in male rats. Pharmazie, 60, 689-695. Mansouri, A., Embarek, G., Kokkalouc, E., Kefalas, P., 2005. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem, 89, 411–420. Marwat, S.K., Khan, M.A., Rehman. F., Bhatti, I.U., 2009. Aromatic plant species mentioned in the Holy Qura’n and Ahadith and their ethnomedicinal importance. Pak. J. Nut. 8, 1472–1479. Maxwell, S.R., Moots, R.J., Kendall, M.J., 1994. Corticosteroids: possible damage to the cardiovascular system. Postgrad. Med. J. 70, 863–870. Mazziotti, G., Gazzaruso, C., Giustina, A., 2011. Diabetes in Cushing syndrome: basic and clinical aspects. Trends Endocrinol. Metab. 22, 499–506. McGavock, J.M., Lingvay, I., Zib, I., Tillery, T., Salas, N., Unger, R., Levine, B.D., Raskin, P., Victor, R.G., Szczepaniak, L.S., 2007. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 16, 1170–1175. Meuleman, J., Katz, P., 1985. The immunologic effects, kinetics, and use of glucocorticosteroids. Med. Clin. North Am. 69, 805-816. Miell, J.P., Englaro, P., Blum, W.F., 1996. Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Horm. Metab. Res. 28, 704–707. Mohamed, E.T., Safwat, G.M., 2016. Evaluation of cardioprotective activity of Lepidium sativum seed powder in albino rats treated with 5-fluorouracil. Beni-Suef Univ. J. Basic Appl. Sci., 5, 208–215. Mokhtari, M., Sharifi, A., Sabzevari Fard, A., 2008. Effects of palm seed alcoholic extract on the blood glucose and lipid concentrations in diabetic male rats. Kurdistan J. Med. Sci. 12, 8-15. Moslehi-Jenabian, S., Pedersen, L.L., Jespersen, L., 2010. Beneficial Effects of Probiotic and Food Borne Yeasts on Human Health. Nutrients 2, 449–473. Murakami, T., Ida, M., Shima, K., 1995. Dexametasone regulates obese expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun, 214,1260–1267. Musa, A.I., 2018. Effect of date palm (Phoenix dactylifera) on lipid profile in experimental rat. IJCRT 6, 2320-2882. Nagpal, R., Kumar, A., Kumar, M., Behare, P.V., Jain, S., Yadav, H., 2012. Probiotics, their health benefits and application for developing healthier foods: a review. FEMS microbial. Lett. 334, 1-15. Nayak, S.K., 2011. Biology of eukaryotic probiotics. In: Probiotics (Liong M-T, ed.). Berlin: Springer, pp. 29–55. Noverraz, M., 1953. Colorimetric determination of the albumin/globulin ratio in blood serum. Schweiz. Med. Wochenschr. 83, 1092-1093. Oie, L., Liong, M., 2010. Cholesterol lowering effects of probiotics and probiotics: A review of in vivo and in vitro findings, International Journal of Molecular Science 11, 2499-2522. Orabi, S.H., Shawky, S.M., 2014. “Effect of date palm (Phoenix dactylifera) seeds extracts on hematological, biochemical parameters and some fertility indices in male rats,” International Journal of Sciences: Basic and Applied Research 17, 137–147. Oyetayo, V.O., Adetuyi, F.C., Akinyosoye, F.A., 2003. Safety and protective effect of Lactobacillus acidophilus and Lactobacillus casei used as probiotic agent in vivo. Afr. J. Biotechnol. 2, 448–452. Pagana, K.D., Pagana, T.J., 2010. Mosby’s Manual of Diagnostic and Laboratory Tests (4th edn.). Mosby Elsevier, St. Louis. Pan, D., Yu, Z., 2013. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5, 108-119. Park, S.H., Hanning, I., Perrota, A., Bench, B.J., Alm, E., Ricke, S.C., 2013. Modifying the gastrointestinal ecology in alternatively raised poultry and the potential for molecular and metabolomic assessment. Poult. Sci, 92, 546-561. Paryad, A., Mahmoudi, M., 2008. Effect of different levels of supplemental yeast (Saccharomyces cerevisiae) on performance, blood constituents and carcass characteristics of broiler chicks, African J. Agricultural. Res. 3, 835-842. Patterson, J.A., Burkholder, K.M., 2003. Application of prebiotics and probiotics in poultry production. Poultry Science 82, 627-631. Randhawa, M., 2008. Black seed, Nigella Sativa, Deserves more attention. J. Ayub Med. Coll. Abbottabad. 20, 15- 18. Rebuffé-Scrive, M., Krotkiewski, M., Elfverson, J., Björntorp, P., 1988. Muscle and adipose tissue morphology and metabolism in Cushing’s syndrome. J. Clin. Endocrinol. Metabol. 67, 1122–1128. Reichardt, H.M., Schutz, G., 1998. Glucocorticoid signalling: multiple variations of a common theme. Mol. Cell Endocrinol.146, 1–6. Ren, R., Oakley, R.H., Cruz-Topete, D., Cidlowski, J.A., 2012. Dual role for glucocorticoids in cardiomyocyte hypertrophy and apoptosis. Endocrinology, 153, 5346–5360. Reul, B.A., Ongemba, L.N., Pothier, A.M., Henguin, J.C., and Brichard, S.M., 1997. Insulin and insulin-like growth factor I antagonize the stimulation of ob gene expression by dexamethasone in cultured rat adipose tissue. Biochem J, 24(Pt 2):605–610. Reitman, S., Frankel, S., 1957. A colorimetric method for determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28, 56–63. Rock, W., Rosenblat. M., Borochov-Neori, H., Volkova, N., Judienstein, S., Elias, M., Aviram, M., 2009. Effect of date (Phoenix dactylifera L., Medjool or Hallawi Variety) consumption by healthy subjects on serum glucose and lipid levels and on serum oxidative status. Journal of Agric. Food Chemistry 57, 8010-8017. Rockall, A.G, Sohaib, S.A., Evans, D., Kaltsas, G., Isidori, A.M., Monson, J.P., Besser, G.M., Grossman, A.B., Reznek, R.H., 2003. Hepatic steatosis in Cushing’s syndrome: a radiological assessment using computed tomography. Eur. J. Endocrinol. 149, 543–548. Satoh, K., 1978. Colorimetric method for determination of malondialdehyde in tissue sample. Clinica Chimica Acta, 90, 37. Schacke, H., Docke, W.D., Asadullah, K., 2002. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 96, 23–43. Schwarz, J.M., Linfoot, P., Dare, D., Aghajanian, K., 2003. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr, 77, 43–50. Sener, G., Sert, G., Ozer Sehirli, A., Arbak. S., Uslu, B., Gedik, N., Ayanoglu-Dulger, G., 2006. Pressure ulcer-induced oxidative organ injury is ameliorated by betaglucan treatment in rats. Int. Immuno pharmacol, 6, 724–732. Seyidoglu, N., Galip, N., Sonat, F.A.K., 2013. Effect of Yeast Culture on Growth Performance, Haematological and Biochemical Indices of New Zealand White Rabbits. Uludag Univ. J. Fac. Vet. Med, 32, 11-17. Shashidhara, R. G., Devegowda, G, 2003. Effect of dietary manna- oligosaccharide on broiler breeder production traits and immunity. Poult. Sci. 82, 1319-1325. Shivashankara, K.S., Isobes, S., Al-Haq, M. I., Takenaka, M., Shinha, T., 2004. Fruit antioxidant activity, ascorbic acid, total phenol, quercetin, and carotene of Irwin mango fruits stored at low temperature after high electric field treatment. J. Agric. and Food Chem. 52, 1281–1286. Silva, V., Lobato, R.V., Andrade, E.F., de Macedo, C.G., Napimoga, J.T., Napimoga, M.H., Pereira, L.J., 2015. β-Glucans (Saccharomyces cereviseae) Reduce Glucose Levels and Attenuate Alveolar Bone Loss in Diabetic Rats with Periodontal Disease. PloS one 10(8), e0134742. Siriken, B., Bayran, I., Onol, A.G., 2003. Effects of probiotics: alone and in a mixture of Biosac plus Zinc Bacitracin on the caecal microflora of Japanese quail. Res. Vet. Sci, 75, 9-14. Situnayake, R., Kitas, G., 1997. Dyslipidemia and rheumatoid arthritis, Ann. Rheum. Dis. 56, 341-342. Slavin, B. G., Ong, J. M., Kern, P.A., 1994. Hormonal regulation of hormone sensitive lipase activity and mRNA levels in isolated rat adipocytes. J. Lipid Res. 35, 1535–1541. Socha, P., Rujner, J., Socha, J., 1992. The role of oxygen radicals and their antioxidans in pathologenesis of chronic hepatitis. Ped. Pol. (Suppl 1-2), 139-144. Spring, P., Wenk, C., Dawson, H.A., Newman, K.E., 2000.The effects of dietary mannan oligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of salmonella challenged broiler chicks. Poult. Sci, 79, 205- 511. Tang, Z.X., Shi, L.E., Aleid, S.M., 2013. Date fruit: Chemical composition, nutritional and medicinal values, products. J. Sci. Food Agric. 93, 2351e2361. Tietz N.W., Burtis C.A., Duncan P., Ervin K., Petitclerc C.J. 1983., A reference method for measurement of alkaline phosphatase activity in human serum. Clin. Chem. 29, 751–761. Trautwein, E.A., Rieckhoff, D., Erbersdobler, H.F., 1995. Dietary inulin lowers plasma cholesterol and triacylglycerol and alters biliary bile acid profile in hamsters. J. Nutrit. 128, 1937–1943. Trementino, L., Arnaldi, G., Appolloni, G., Daidone, v., Scaroni, C., Casonato, A., Boscaro, M., 2010. Coagulopathy in Cushing’s syndrome. Neuroendocrinology 92 (Suppl), 55–59. Trinder, P., 1969. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. 22, 158-161.
Vayalil, P.K., 2002. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae). Journal of Agriculture and Food Chemistry 50, 610–617(s). Volman, J. J., Ramakers, J. D., Plat, J., 2008. Dietary modulation of immune function by beta-Dglucans. Physiology and Behavior 94(2), 276-284. Wan Ismail, I.W., Mohd Radzi, M.F., 2013. Evaluation on the Benefits of Date Palm (Phoenix dactylifera) to the Brain. Altern. Integ. Med. 2, 4. Weinstein, S.P., Wilson, C.M., Pritsker, A., Cushman, S.W., 1998. Dexamethasone inhibits insulin-stimulated recruitment of GLUT4 to the cell surface in rat skeletal muscle. Metab. Clin. Exp. 47, 3–6. Wajchenberg, B.L., 2000. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 21, 697–738. Wojcik, R., 2010. Effect of Brewer,s yeast (Saccharomyces cerevisiea) extract on selected parameters of humoral and cellular immunity in lambs. Bull. Vet. Inst. Pulawy 54, 181-187. Wolf, P.L., 1999. Biochemical diagnosis of liver disease. Indian Journal of Clinical Biochemistry 14, 59-90. Wu, Y., Wu, Q., Zhou, Y., Ahmad, H., Wang, T., 2013. Effects of clinoptilolite on growth performance and antioxidant status in broilers. Biological Trace Element Research 155, 228–235. Xue, Q., Patterson, A.J., Xiao, D., Zhang, L., 2014. Glucocorticoid Modulates Angiotensin II Receptor Expression Patterns and Protects the Heart from Ischemia and Reperfusion Injury. PloS one 9, e106827. Zangiabadi , N., Asadi-Shekaari , M., Sheibani, V., Jafari, M., Shabani, M., Asadi, A. R., Tajadini, H., Jarahi, M., 2011. Date Fruit Extract Is a Neuro Protective Agent in Diabetic Peripheral Neuropathy in Streptozotocin-Induced Diabetic Rats: A Multimodal Analysis. Oxid. Med. Cell Longev. 2011, 976948. Zhang, A.W., Lee, B.D., Lee, S.K., Lee, K.W., An, G.H., Song, K.B., Lee, C.H., 2005. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult. Sci. 84, 1015-1021. Zhao, L.F., Iwasaki, Y., Zhe, W., Nishiyama, M., Taguchi, T., Tsugita, M., Kambayashi, M., Hashimoto, K., Terada, Y., 2010. Hormonal regulation of acetyl-CoA carboxylase isoenzyme gene transcription. Endocr. J. 57, 317–324. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|