|
|
Journal of Advanced Veterinary Research Volume 10, Issue 1, 2020, Pages: 13-16 www.advetresearch.com |
|
|
Osteoarthritic Synovial Derived Stem Cells Augmented with Subchondral Drilling for Repair of Large Osteochondral Defects in Rabbit Model |
|
Amany Sayed Mawas1, Elhussein Elbadry Mahmoud2* |
|
1Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt. 2Department of Surgery, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt. |
|
|
|
Received: 7 November 2019; Accepted: 21 December 2019 (*: Corresponding author: E.badry@vet.svu.edu.eg) |
|
Abstract |
|
|
|
This study showed the effectiveness of combination therapy of osteoarthritic synovial derived stem cells (OA-SDSCs) with subchondral drilling for large osteochondral defects repair in mature rabbit model. The defect was created at load-bearing area of the medial femoral condyle of both knees (6 mm length × 3 mm width). Then, mature rabbits were separated randomly into 2 groups: 3 subchondral holes were penetrated the subchondral bone in the defect site (drilled group), and then an intra-articular injection of one million OA-SDSCs into the knee joint was performed (combined group). After two months, rabbits were euthanized to perform histological assessment of the repaired tissue using safranin O stain. Repaired tissue was visually more whitish in the drilled group than in the combined group. Histologically, repaired tissue almost revealed fibrocartilage with subchondral repair in the combined group. However, fibrous tissue was represented in the drilled group. On Pineda score, the combined group was significantly better than of the drilled group (P = 0.001). Finally, using of OA-SDSCs with subchondral drilling promotes better cartilage repair than using subchondral drilling alone. |
|
|
|
Keywords: Subchondral drilling, OA-SDSCs, Osteochondral repair, Rabbit model |
|
|
|
Introduction |
|
|
|
Healing of the articular cartilage lesions is potentially limited because of its poor vascularization. Although bone marrow stimulation such as subchondral drilling is the most common traditional surgical treatment option for cartilage lesions (Pridie, 1959). However, its clinical outcome is not satisfactory due to the formation of fibrous tissue or fibrocartilage (Knutsen et al., 2004) which does not resemble the surrounding hyaline cartilage. The subchondral drilling has aimed to clot formation through blood migration containing mesenchymal stem cells (MSCs) from the bone marrow into the defect site (Madry et al., 2010). MSCs have the ability to differentiate into chondrocytes, osteoblasts, and adipocytes (Pittenger et al., 1999), which participate in fibrocartilage formation due to their low concentration in the bone marrow. Most of the previous studies have used monolayer cultured bone marrow MSCs with subchondral drilling to promote osteochondral repair than using subchondral drilling alone in rats (Nishimori et al., 2006), and goats (Nam et al., 2013). A new source of MSCs from synovial tissue has recorded, and named synovial MSCs. These cells are a good option for cartilage repair because of their high proliferative capacity and chondrogenic potential (Sakaguchi et al., 2005), and easy harvesting synovial tissue arthroscopically with low degree of minimal invasiveness (De Bari et al., 2001). Although, Interleukin (IL) -1β has been shown to trigger pathological processes in rheumatoid arthritis and osteoarthritis (OA), the proliferation rate and chondrogenic potency of synovial MSCs were improved after treatment with IL-1β (Matsumura et al., 2017). For this reason, we hypothesized that osteoarthritic synovial derived stem cells (OA-SDSCs) have the potential to enhance chondrogenic capacity, maintain proliferative property, and have improved quality of the repaired articular cartilage. Hence, this study was aimed to examine the impact of an intra-articular injection of OA-SDSCs with subchondral drilling for large osteochondral defects repair in of the mature rabbit model. |
|
|
|
Materials and methods |
|
This study was approved by ethical committee of research facility (Faculty of Veterinary Medicine, South Valley University, Egypt. Isolation and expansion of OA-SDSCs The synovial tissue was collected from the medial aspect of the osteoarthritic knee joint of the rabbit for isolation of OA-SDSCs using explant method. In brief, synovial tissue was divided into tiny pieces and cultured on culture dishes, and OA-SDSCs started to migrate from the periphery of the tissue and strongly adhered to culture dishes to form colonies. These OA-SDSCs were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Gibco®, Life Technologies, USA) containing 10% deactivated fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO) and 1% antibiotics (penicillin and streptomycin) (Invitrogen, CA, USA). Then, the tissue was removed after 1 week. Cells were seeded on 10-cm2 tissue culture dishes (BD Falcon™; BD Biosciences, Franklin Lakes, NJ), and incubated in a humidified atmosphere with 5% Co2 at 37˚C. After 2 weeks, 0.25% trypsin (Gibco®, Thermo Fisher Scientific, Waltham, MA) was used for 2 minutes for cell detachment, the cells were subcultured under the same conditions. These adherent cells at first passage are referred to as OA-SDSCs that were used in this experiment (Mahmoud et al., 2019b). Intra-articular injection of OA-SDSCs into the knee joint In the present study, ten male white rabbits, above 8 months of age and weighing 3.0-3.49 kg, were kept in separate cages freely. An intravenous infusion of pentobarbital (30 mg/kg body weight) was performed for induction of anesthesia (Mahmoud et al., 2019a). With lateral patellar dislocation, medial parapatellar surgical approach was used to expose the knee joint. Using an electric drill, bilateral osteochondral defects (Length 6 mm; width 3 mm) were created on the weight-bearing portion of the medial femoral condyles. Then the 20 rabbits' knees were randomly divided into 2 groups equally: Group 1 (the drilled group), three holes were drilled for subchondral perforation by 0.5 mm Kirschner wire (n=10). However, in group 2 (the combined group), three holes were drilled for subchondral perforation with an intra-articular injection of 1×106 OA-SDSCs (n=10), then the joint capsule was closed in routine manner. Macroscopic and histological assessment of the repaired tissue At 2 months post-treatment, rabbits were euthanized by intravenous injection of a lethal dose of pentobarbital (180 mg/kg body weight). The femoral condyles were collected for macroscopical evaluation, fixed in a 4% paraformaldehyde phosphate buffered solution (PFA) for 48 hours, and then decalcified with 10% ethylenediaminetetraacetic acid solution (EDTA) (Nacalai Tesque, Inc.) for 10 weeks. The samples were prepared in paraffin sections and cut into 5-µm-thick sagittal sections which were stained with Safranin O/fast green stain for histological scoring using Pineda score graded with 0 point as the best and 14 points as the worst (Table 1) (Pineda et al., 1992). Statistical analysis The histological scoring was analyzed by using of Mann-Whitney' U-test, with a 95% confidence interval. Differences of P < 0.05 were considered significant. |
|
|
|
Results |
|
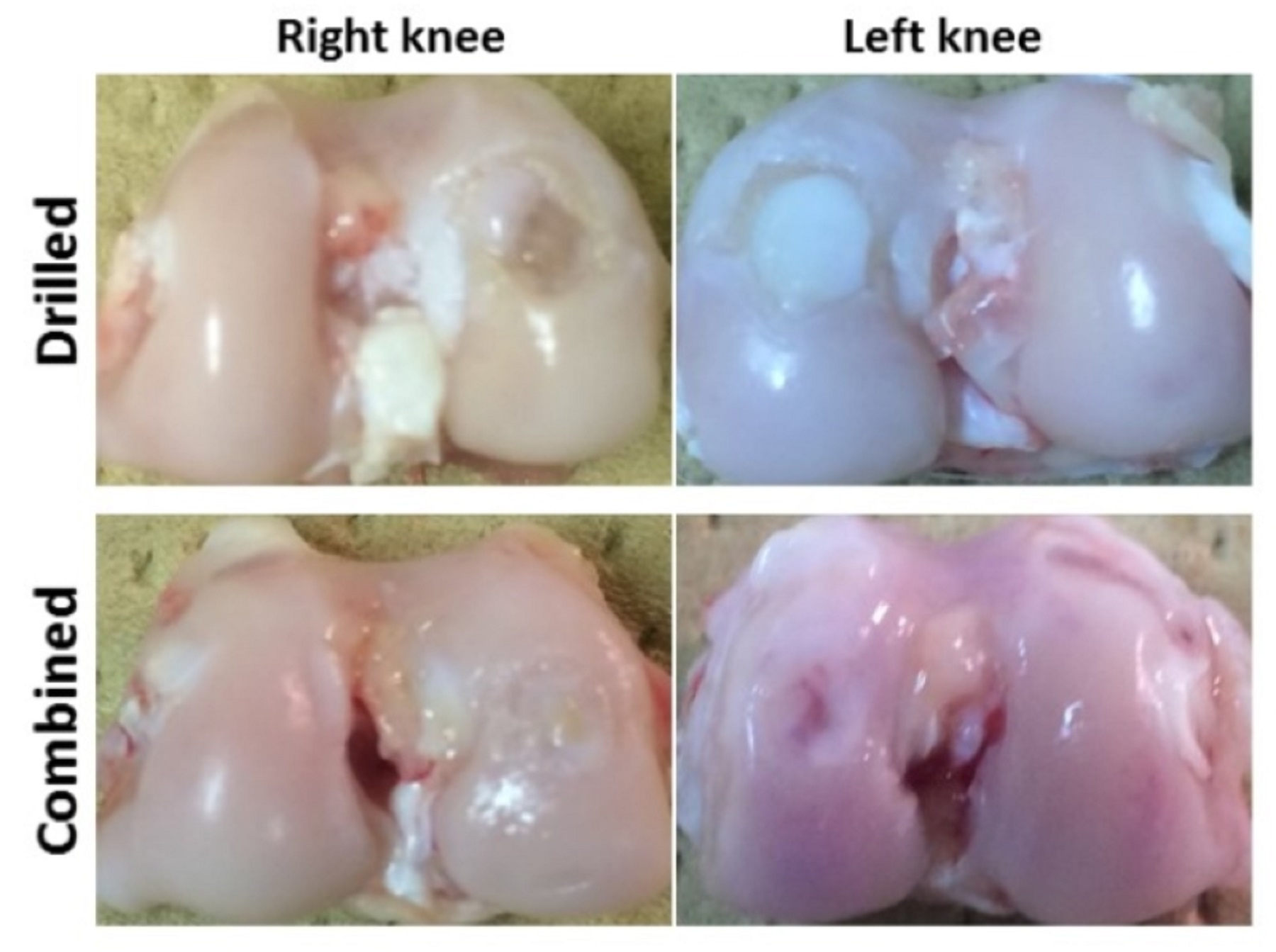
Macroscopic findings In the drilled group, repair tissue was soft and more whitish than normal with easily identifying the defect margins. While in the combined group, repair tissue was somewhat resembled the normal one with recognizing defect margins (Fig. 1).
Fig. 1. Representative macroscopic findings of right and left knees were indicated a good condition of the reparative tissue in case of combined group when compared with the drilled group. Histological findings and scoring In the drilled group, fibrous tissue was formed in the defects instead of the subchondral bone and cartilage. In the combined group, complete repair of the subchondral bone at the same level of the adjacent normal tissue was confirmed, which covered by fibrocartilage containing chondrocytes or hyaline cartilage. In addition, integration of the reparative tissue was good with the surrounding native tissue. At 2 months, the histological scoring results were 10.3±0.5 and 7.9±1.5 for the drilled group and the combined group, respectively. On Pineda score, the histological score was significantly better in the combined group than in the drilled group (P= 0.001) (Fig. 2).
Fig. 2. A. Representative microscopic findings using Safranin O/ Fast green stain. There was a little fibrous tissue inside the defect in case of the drilled group. While complete repair of the subchondral bone covered by Hyaline/fibrocartilage including chondrocytes presented in case of the combined group (original magnification: 40X). B. On Pineda histological scoring, combined group was significantly histologically better than of the drilled group. *p < 0.05. |
|
|
|
Discussion |
|
The hypothesis of this study was consistent with the obtained results, which revealed that OA-SDSCs augmented subchondral drilling for better repair of large osteochondral defect of the medial femoral condyle in the mature rabbit model, although quality of the reparative tissue was not perfect. Up till now, one of the common surgical technique for cartilage repair is subchondral drilling leading to the formation of fibrous tissue, or fibrocartilage with inferior quality than native hyaline cartilage by inducing of the bleeding for recruitment of bone marrow mesenchymal stromal cells from the subchondral bone into the defect site (Frisbie et al., 2003). In the present study, fibrous tissue was formed in the large osteochondral defects in case of drilled group, which may be attributed to the traumatic inflammatory response, and less bleeding from subchondral holes leading to carrying low number of bone marrow stromal cells, which were not enough to restore the large defect. So, it is not good option for using subchondral drilling alone for repair of large osteochondral defect. Previous experimental studies for cartilage repair depended upon using subchondral drilling with atelocollagen membrane (Hamanishi et al., 2013), and scaffolds such as chitosan (Merchand et al., 2012). But, the coverage of the defect by scaffold inhibited cartilage repair. In this study, authors tried to improve quality of the productive repaired tissue through combination therapy of OA-SDSCs with subchondral drilling which confirmed by complete repair of the subchondral bone covered by fibrocartilage containing chondrocytes with good integration with the surrounding native tissues. More importantly, SDSCs have been successfully isolated and expanded from pathological synovial tissue from RA or OA patients, providing for a good option for cartilage regeneration/repair (Nagase et al., 2008). |
|
|
|
Conclusion |
|
An intra-articular injection of OA-SDSCs improved quality of the reparative tissue produced by subchondral drilling alone. Large number of OA-SDSCs may be more effective for treating large defects. Therefore, future research trials of combination therapies of different densities of OA-SDSCs with surgical procedures such subchondral drilling to form neo-cartilage like tissues are required with long-term durability. |
|
|
|
Conflict of Interests |
|
|
|
The authors declare no conflict of interest. |
|
|
|
References |
|
|
|
De Bari, C., Dell'Accio, F., Tylzanowski, P., Luyten, F.P., 2001. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 44, 1928-1942. Frisbie, D.D., Oxford, J., Southwood, L., Trotter, G.W., Rodkey, W.G., Stedman, J.R., Goodnight, J.L., Mcllwraith, C.W., 2003. Early events in cartilage repair after subchondral bone microfracture. Clin. Orthop. 407, 215-227. Hamanishi, M., Nakasa, T., Kamei, N., Kazusa, H., Kamei. G., Ochi, M., 2013. Treatment of cartilage defects by subchondral drilling combined with covering with atelocollagen membrane induces osteogenesis in a rat model. J. Orthop. Sci. 18, 627-635. Knutsen, G., Engebretsen, L., Ludvigsen, T.C., Drogset, J.O., Grontvedt, T., Solheim, E., Strand, T., Roberts, S., Isaksen, V., Johansen, O., 2004. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J. Bone Joint Surg. Am. 86, 455-464. Madry, H., van Dijk, C.N., Mueller-Gerbl, M., 2010. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. 18, 419-433. Mahmoud, E.E., Adachi, N., Mawas, A.S., Deie, M., Ochi, M., 2019a. Multiple intra-articular injections of allogeneic bone marrow-derived stem cells potentially improve knee lesions resulting from surgically induced osteoarthritis: an animal study. Bone Joint J. 101-B, 824-831. Mahmoud, E.E., Adachi, N., Mawas, A.S., Gaarour, O.S., Ochi, M., 2019b. Coculturing of mesenchymal stem cells of different sources improved regenerative capability of osteochondral defect in the mature rabbit: An in vivo study. J. Orthop. Surg. (Hong Kong) 27, 2309499019839850. Matsumura, E., Tsuji, K., Komori, K., Koga, H., Sekiya, I., Muneta, T., 2017. Pretreatment with IL-1β enhances proliferation and chondrogenic potential of synovium-derived mesenchymal stem cells. Cytotherapy 19, 181-193. Merchand, C., Chen, G., Tran-Khanh, N., Sun, J., Chen, H., Buschmann, M., Hoemann, C.D., 2012. Microdrilled cartilage defects treated with thrombin-solidified chitosan/blood implant regenerate a more hyaline, stable, and structurally integrated osteochondral unit compared to drilled controls. Tissue Eng. Part A. 18, 508-519. Nagase, T., Muneta, T., Ju, Y.J., Hara, K., Morito, T., Koga, H., Nimura, A., Mochizuki, T., Sekiya, I., 2008. Analysis of the chondrogenic potential of human synovial stem cells according to harvest site and culture parameters in knees with medial compartment osteoarthritis. Arthritis Rheum. 58, 1389-1398. Nam, H.Y., Karunanithi, P., Loo, W.C.P., Naveen, S.V., Chen, H.C., Hussin, P., Chan, L., Kamarul, T., 2013. The effects of staged intra-articular injection of cultured autologous mesenchymal stromal cells on the repair of damaged cartilage: a pilot study in caprine model. Arthritis Res. Ther. 15, R129. Nishimori, M., Deie, M., Kanaya, A., Exham, H., Adachi, N., Ochi, M., 2006. Repair of chronic osteochondral defects in the rat. J. Bone Joint Surg. Br. 88-B, 1236-1244. Pineda, S., Pollack, A., Stevenson, S., Goldberg, V., Caplan, A., 1992. A semiquantitative scale for histologic grading of articular cartilage repair. Acta. Anat. 143, 335-340. Pittenger, M.F., Mackay, A.M., Beck, S.C., Jaiswal, R.K., Douglas, R., Mosca, J.D., Moorman, M.A., Simonetti, D.W., Craig, S., Marshak, D.R., 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143-147. Pridie, K.H., 1959. A method of resurfacing osteoarthritic knee joints. J. Bone Joint Surg. Br. 41, 618-619. Sakaguchi, Y., Sekiya, I., Yagishita, K., Muneta, T. 2005. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 52, 2521-2529. |
|
|