|
|
Journal of Advanced Veterinary Research Volume 10, Issue 1, 2020, Pages: 21-28 www.advetresearch.com |
|
|
Gastrointestinal Helminthic Infections in Egyptian Domestic Camels, Camelus dromedarius, with a Special Reference to Trichostrongylids |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Khaled M. El-Dakhly*, Waleed M. Arafa, Lilian N. Mahrous, Alaa M. Yousef |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Department of Parasitology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Received: 03 December 2019; Accepted: 01 January 2020 (*: Corresponding author: eldakley_s71@yahoo.com) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
In Egypt, scare literature explored the coprological examination of domestic camels. Therefore, a total of 626 fecal samples from domestic dromedaries, Camelus dromedaries, permitted to slaughtering in El-Warrak abattoir, Giza were taken. Coproparasitological investigations including sedimentation and floatation techniques, fecal culture and larval identification were done. The overall prevalence of parasitic infections was 41.53%. Fifteen species of helminth eggs/protozoan oocysts were recovered. The prevalence of helminths was 28.11% (176/626) and that of protozoa was 5.59% (35/626). Mixed infections were reported in 7.82% (49/626) of camels. The revealed trematode was Fasciola sp. (1.12%), tapeworms belonged to Anoplocephalids (5.27%), protozoan oocysts were Eimeria cameli, E. dromedarii, E. rajasthani (11.02% for all Eimeria spp.) and Buxtonella sp. (0.32%). The recovered nematodes belonged to Trichuris sp. (1.92%) and trichostrongyles (31.0%). Coproculture of the later revealed the presence of 8 species; Trichostrongylus axei, Tr. colubriformis, Chabertia ovina, Ostertagia ostertagi, Haemonchus sp., Oesophagostomum sp., Bunostomum sp. and Nematodirus sp. Morphometric characteristics of larvae were recorded. Age and seasonal variations revealed significant (P≤0.05) differences among examined camels. Animals aged more than 5 years had the highest infections rate (45.96%; 199/433) and nematodes were the significantly (P≤0.05) predominant. In winter, the highest prevalence (60.67%; 108/178) was recorded. Oppositely, sex had no significant differences. Due to the expected important role played by imported camels in transmitting various parasitic infections, veterinarians and parasitologists are extremely advised to apply further studies on the helminth fauna, particularly gastrointestinal nematodes, of both domestic and imported camels, by the use of traditional and molecular tools. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Keywords: Helminths, Strongyles, Seasons, Prevalence, Camels, Egypt |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Introduction |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Domesticated camels (dromedaries or one humped camels, Camelus dromedarius) are the largest species among camelids. The terminology dromedary is initiated form a wide spread Greek word, dromos, that means road, reflecting basically both riding and racing (Radfar and Gowhari, 2013). Those animals are economically potential for countries encountered with their breeding. They are multipurpose animals i.e. they almost used for transport, meat and milk. Moreover, camel racing is a traditional sport in countries of the Arabian Peninsula, like Oman and the United Arab Emirates, and northern Africa, like Morocco. In these countries, they are commonly used by Bedouins in their long tribes (Wernery and Kadden, 2002; Khatami, 2016; Dubey and Schuster, 2018). In Egypt, according to 1998 statistics, the number of camel populations was approximately 95,000 (Saber, 1998). Over the years, the number is declining, since camels are no longer, except in rare instances, used for transport as well as their being a protein source becomes limited. Recently, in 2016, the estimated camel population in the countryside was 66,228. Surprisingly, approximately 176,000 heads are annually imported, mostly, from East African countries mainly, Ethiopia and Sudan (Elfadaly, 2016). Among the significant obstacles of the camel population production, is the parasitic infection, particularly with gastrointestinal helminths and coccidian oocysts. The majority of those parasites are responsible for the parasitic gastroenteritis (Parsani et al., 2008; Radfar and Gowhari, 2013). Gastrointestinal helminthic infections of domesticated camels may be either common or occasional. Some helminths, like Nematodirus dromedarii and Haemonchus longistipes, are camels-specific, while others, like Trichostrongylus spp., Chabertia spp., Oesophagostomum venulosum, Cooperia oncophora, Ostertagia ostertagi, which infect a variety of domestic and wild animals, may be easily access to camels via the grazing habits on the same pastures. Among all, Haemonchus spp. are the most pathogenic with a significant impact and economic losses of the camel industry (Banaja and Ghadour, 1994; Wernery and Kadden, 2002; Parsani et al., 2008; Radfar and Gowhari, 2013). The pathogenic effect of gastrointestinal helminths is optimized by a massive infection that reduces the absorption of nutrients and increases the tissue damage. Simultaneously, helminth parasites not only reduce the productivity, quantitatively and qualitatively, and performance of camels but also predispose them to other infections (Rewatkar et al., 2009; Mouldi et al., 2015). Due to being that most of gastrointestinal helminths infecting camels are detected only during post mortem in abattoirs (Borji et al., 2010), as well as the rearing habits by owners who prefer breeding in free-ranging systems, thus away from the veterinary care, it seems that the present surveillances and questionnaires are extremely insufficient. Therefore, the current study was conducted to explore the gastrointestinal helminthic infections, particularly nematodosis, in domestic camels in various localities in Egypt via coprological examination and identification of the recovered nematode species. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Materials and methods |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Study area and sampling Fecal samples were collected from 626 one-humped camels (Camelus dromedarius) of both sexes at different ages during the period from March 2018 to March 2019 admitted to slaughtering in El-Warrak abattoir, Giza province (coordinates: 30° 1′ 0″ N, 31° 13′ 0″ E), Egypt. Fresh samples were taken directly from the rectum of slaughtered camels by disposable rectal gloves. Each sample was placed in clean universal plastic bags and labeled by data as age and sex then transported in an ice box to the laboratory of Parasitology, Faculty of Veterinary Medicine, Beni-Suef University, Egypt. Coprological examination The collected samples were examined using simple sedimentation method for trematodes eggs and flotation method for eggs of nematodes, tapeworms and coccidian oocysts according to the method described by Soulsby (1982) and Markel et al. (1999). Fecal culture and harvesting trichostrongylid larvae The pooled camelid fecal pellets were large-sized, so they were thoroughly crumbled before being mixed with water to produce more or less pasty mixture, which is slightly compacted, in a depth of about 5 cm in wide glass jars of approximately 1 L capacity. A hole is left in the centre of the culture by holding a stamper vertically in the centre of the jar, leaving the mixtures lightly compacted around it. The culture was sufficiently moistened to avoid dryness while being incubated, but without being water-logged. Thereafter, jars were incubated in the dark at 26-28 °C for 5-7 days, during which it was periodically checked and moistened if needed. After the end of the incubation period, the inside of the culture is slightly sprayed with water before being placed in bright light that stimulates third larval stages to migrate up the inner surfaces of the jars’ walls. The culture was repeatedly harvested over several days by holding the jar sloped with the mouth pointing downwards and then spraying the inner walls and allowing the larval suspension to drain into suitable containers (Zajac and Conboy, 2006; van Wyk and Mayhew, 2013). Larval preparation In order to retain the intact outline, translucent appearance and the intact internal structure, larval suspension is preserved in 1-2% formalin and heat at 56ºC for 60 sec. Then, a drop of the larval suspension was put on a clean and dry glass slide for microscopy (van Wyk et al., 2004; van Wyk and Mayhew, 2013). Larval identification Morphological identification of parasitic neamtodes, particularly trichostrongylids, is mainly based on the characterization of larval anterior and posterior ends, the whole larval length and the number of gut cells. The shape of the oesophagus and the presence of anterior refractile bodies are needed for some species (McMurtry et al., 2000; van Wykand Mayhew, 2013). Upon the identification of larvae, the tip of the anterior extremity of a larva is referred to as ‘head’ and the posterior extremity as ‘tail’ and the free sheath beyond the tail tip as the sheath tail extension (STE). The latter is a unique diagnostic feature for the accurate and appropriate identification of nematodal larvae. Larval photographing Available photographs of larvae were taken using a digital microscope (Leica microsystems, CH-9435 Heebrugg, Ec3, Singapore). Measurements of the recovered larvae were in micrometers (El-Dakhly et al., 2012). Statistical analysis Data analysis was performed using a Microsoft Excel worksheet for Windows 2010. Data were summarized by descriptive statistics for the overall prevalence in camels. The Chi square test was used to analyze the effect of risk factors; age, sex and seasons, on the overall parasitic infections. Variables were significant at P≤ 0.05. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Results |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
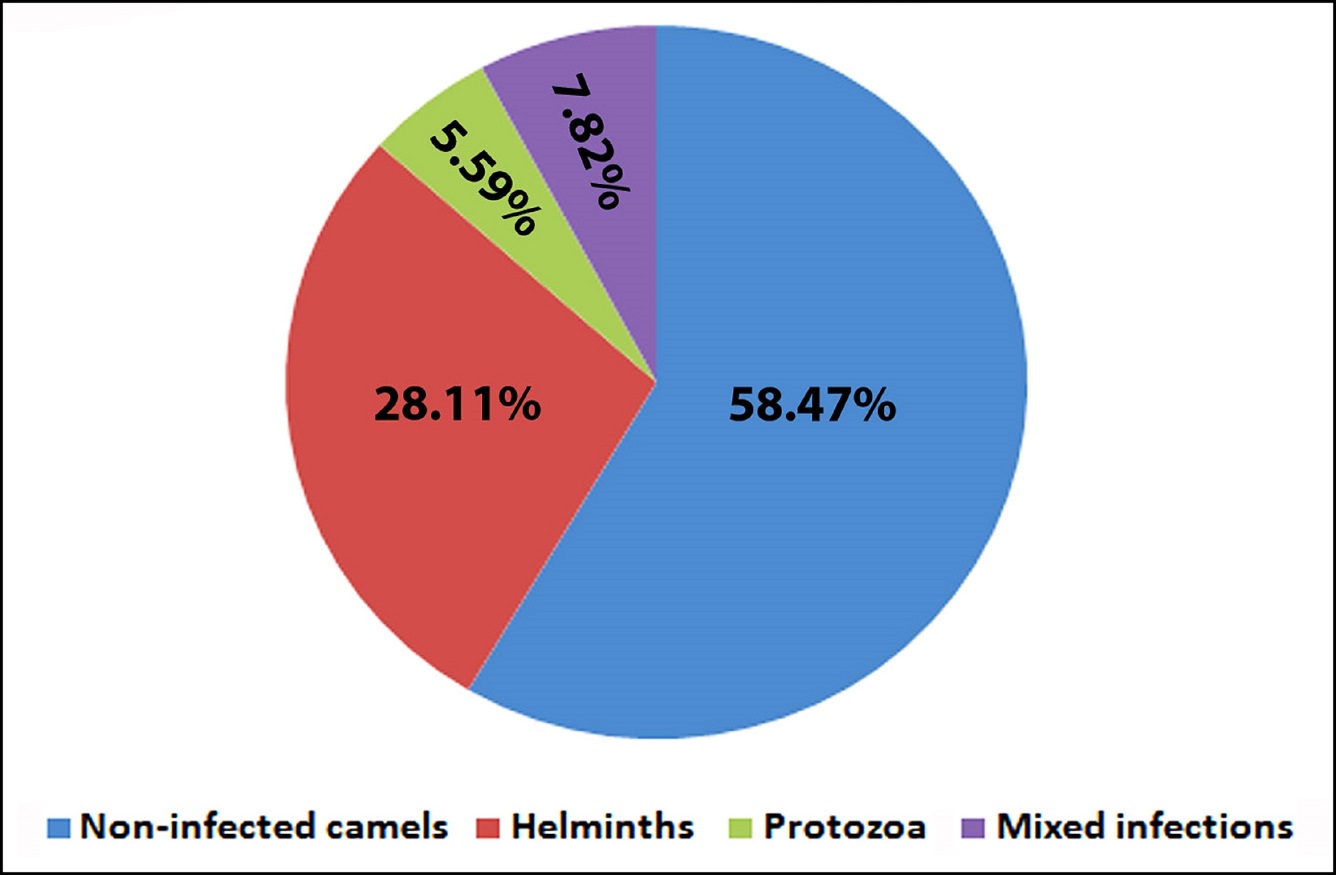
It has been found that the overall infection rate was 41.53% (260/626). Fifteen species of both helminth eggs and protozoan cysts were recovered. Helminth eggs were detected in 28.11% (176/626) of the examined fecal samples. The protozoan oocysts were found in 5.59% (35/626). Meanwhile, mixed infections of both helminths and protozoa were seen in 7.82% (49/626) (Table 1 and Fig. 1). Among helminths, the prevalence of trichostronglye eggs was 31.0% and that of Trichuris sp. was 1.92%. Tapeworm eggs, belonged to anoplocephalids (mostly Moniezia spp.), were recorded in 5.27%, while trematode eggs (Fasciola sp.) could be detected in 1.12%. Eimeria spp. (E. cameli, E. dromedarii and E. rajasthani) oocysts were observed in 11.02% and Buxtonella sp. was recorded in percent of 0.32% of examined specimens. Meanwhile, the infection with both nematodes and Eimeria spp. were detected in 4.95% (31/626). The infection with tapeworms and nematodes were found in 1.27% (8/626). Both tapeworms and Eimeria spp. were seen in 0.95% (6/626) of examined specimens. Similarly, the infection by helminths and protozoa were revealed in 0.31% (2/626). Trematodes and tapeworms were recorded in 0.15% (one/626). Also, the mixed infection by trematodes and nematodes were found in 0.15% (one/626). Table 1. The overall prevalence of parasitic infections among the examined camels
No.: Number of infected animals; %: Percentage of infection
Fig. 1. The overall of parasitic infections among domestic camels. Concerning the age, it has been recorded that camels less than three years had the lowest infection rate. Among those, nematode helminths were identified in 20.0% (7/35). Protozoa were recovered in 5.71% (2/35), mixed infections were detected in 5.71% (2/35), trematode parasites were reported in 2.86% (one/35) and tapeworms were recorded in 2.86% (one/35) of examined camelids. Among animals aged 3-5 years, 17.72% (28/158) had nematodosis. Mixed infections were observed in 5.06% (8/158). Meanwhile, protozoan oocysts were revealed in 4.43% (7/158), tapeworms were recorded in 2.53% (4/158) and trematodes were determined in 0.63% (1/158). Camels aged more than 5 years had the highest infection. Among those, nematodes were observed in 27.48 % (119/433), mixed infections were found in 9.0% (39/433), protozoan oocysts were detected in 26/433 (6.0%), tapeworms were recovered in 2.77% (12/433) and trematodes were identified in 3.0% (3/433). There was a significant difference of the total parasitic infections (Chi-square value was 11.86 at P value 0.0026), particularly nematodes (Chi-square value was 6.37 at P value 0.04) (Table 2). Table 2. The prevalence of helminth and/or coccidian parasites in examined camels relative to the age.
No. = Number of infected camels % = Percentage of infection *: Significant at P ≤ 0.05 Regarding sex, infection rates in male camels with helminths and/or coccidian oocysts were (22.90%; 112/489), (8.38%; 41/489), (5.93%; 29/489), (2.86%, 14/489) and (0.61%; 3/489) for nematodes, mixed infections, protozoal oocysts, tapeworms and trematodes, respectively. Moreover, prevalences in she-camels were (30.66%; 42/137), (5.84%; 8/137), (4.38%; 6/137), (2.19%; 3/137) and (1.46%; 2/137) for nematodes, mixed infections, protozoal oocysts, tapeworms and trematodes, respectively. Based on the sex, there was non-significant infection rate difference in camels (Table 3). Table 3. The prevalence of helminth and/or coccidian parasites in camels relative to sex.
No. = Number of infected camels % = Percentage of infection Significance at P ≤ 0.05 The effect of seasonal variation on the parasitic infection revealed that the highest prevalence was found in winter followed by autumn and spring, and then began to decline in summer. In winter, nematodes were the highest (31.46%; 56/178), mixed infections were reported in 15.73% (28/178), protozoan oocysts were seen in 10.11% (18/178), tapeworms were recorded in 3.37% (6/178) and trematodes were absent. In autumn, infection rates were 30.22% (68/225), 4.0% (9/225), 1.78% (4/225), 2.67% (6/225) and 0.44% (one/225) for nematodes, mixed infections, protozoan oocysts, tapeworms and trematodes, respectively. In spring, nematodes were revealed in 21/106 (19.81%), mixed infection were recovered in 9/106 (8.49%), protozoan oocysts in 8/106 (7.55%) and tapeworms in one/106 (0.94%). However, trematodes were not recorded. In summer, nematodes were obtained in 7.69% (9/117), protozoan oocysts in 4.27% (5/117), tapeworms in 3.42% (4/117), trematodes in 3.42% (4/117) and mixed infections were elucidated in 2.56% (3/117). Statistically, significant differences were detected in the total parasitic infection (Chi-square was 47.97 at P value 0.00001), nematodes (Chi-square was 31.3 at P value 0.00001), trematodes (Chi-square was 4.7 at P value 0.029), protozoa (Chi-square was 14.24 at P value 0.002) and mixed infections (Chi-square was 24.53 at P value 0.00001) (Table 4). Table 4. The prevalence of helminth and/or coccidian parasites in camels relative to seasonal variation.
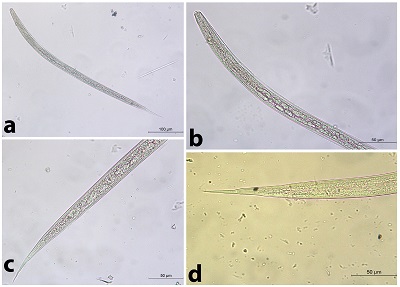
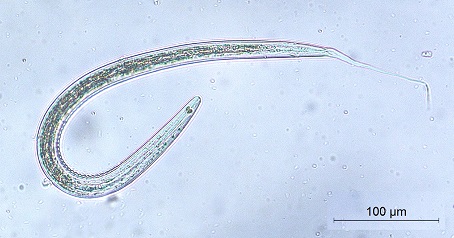
No. = Number of infected camels % = Percentage of infection Significance at P ≤ 0.05 Among the recovered nematodes, it has been found that the application of fecal culture on the obtained fecal specimens revealed the presence of 8 trichostrongylid species, namely Trichostrongylus axei, Tr. colubriformis, Chabertia ovina, Ostertagia ostertagi, Haemonchus sp., Oesophagostomum sp., Bunostomum sp. and Nematodirus sp. Trichostrongylus axei (Fig. 2 a-c) The total length was 557.14-628.57 µm. It was a short and straight. The head was rounded and the gut had 16 intestinal cells. The tail had a simple and blunt terminal end. It had a short STE ranged from 28.57-42.85 µm without a filament. Tr. colubriformis (Fig. 2 a, b, d) Similarly, it was more or less closely related to Trichostrongylus axei, but the tail end had 2-3 tubercles (bifid structure).
Fig. 2. The 3rd larval stage of Trichostrongylus spp. a) The whole larva of Tr. axei. b) The Anterior part of Trichostrongylus spp. c) The posterior part of Tr. axei. d) The posterior part of Tr. colubriformis. Chabertia ovina (Fig. 3) Long larva (the total length is 800 µm). The head was square-shaped. It had 28-32 intestinal cells which were rectangular-shaped. It hada long thin tail sheath (133.5 µm long). The filament was medium-sized measuring approximately 30% of the tail sheath long).
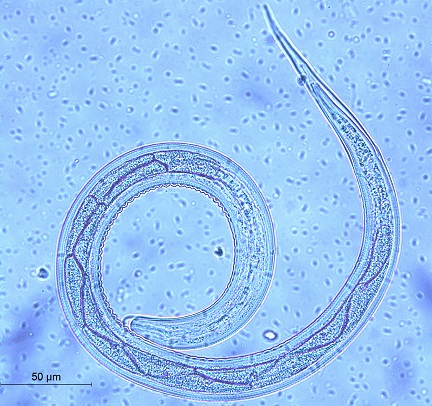
Fig. 3. The 3rd stage larva of Chabertia ovina. a) The anterior part. b) The posterior part. Ostertagia ostertagi (Fig. 4) The total length was 671.4 µm. The head was rounded. It contained 16 intestinal cells. It had a short tail sheath (57.14 µm) and blunt tail tip without a filament. Fig. 4. The 3rd stage larva of Ostertagia ostertagi. Haemonchus sp. (Fig. 5) A medium-sized larva (the total length ranged from 571.42-685.71 µm). The head was bullet. It had 16 alternating zigzag-shaped intestinal cells. It had a medium tail sheath (71.4 µm long). The tail was pointed. The filament constituted 10-15% of the total tail sheath (7.1µm long).
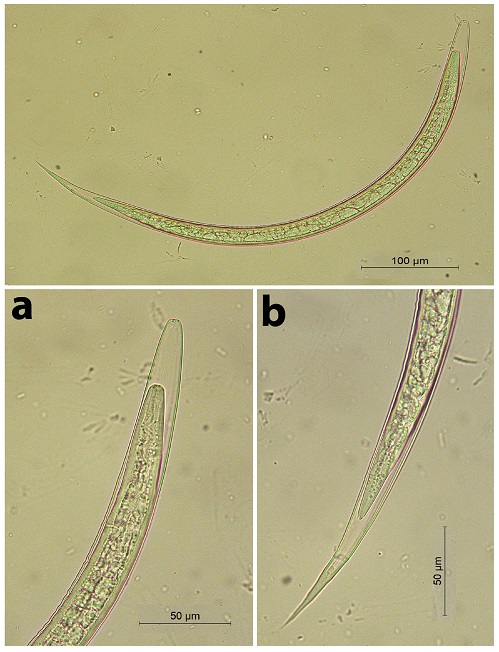
Fig. 5. The 3rd stage larva of Haemonchus sp. a) The whole larva. b) The posterior end Oesophagostomum sp. (Fig. 6) The total length ranged from 714.28-800 µm (long larva). The head was rounded. It had approximately 22 triangular-shaped intestinal cells. It had a long thin tail sheath (it ranged from 128.5-142.8 µm). The larva terminated in a simple point. The filament was long (73 µm long, approximately 60% of STE).
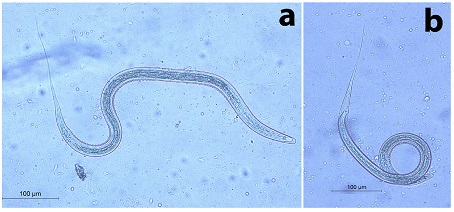
Fig. 6. The 3rd stage larva of Oseophagostomum sp. Bunostomum sp. (Fig. 7) A medium-sized larva (the total length was 628.57 µm). The head was bullet and it had 16 intestinal cells. It had along thin tail sheath measuring 85.7 µm. It had a wide body. The filament was long, measuring 42.85 µm (approximately 50% of STE).
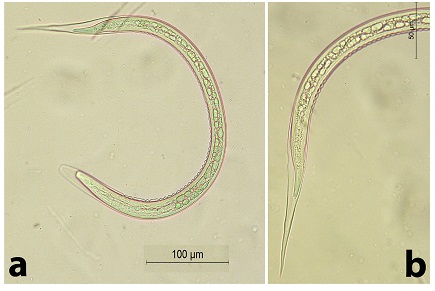
Fig. 7. The 3rd stage larva of Bunostomum sp. a) The anterior part. b) The posterior part. Nematodirus sp. (Fig. 8) A very long larva, measured 885.0 µm. It had a rounded head. STE measured 214.28 µm. The terminal end of the tail was notched (fish-like). The filament measured approximately 100 µm (46.7% of the STE).
Fig. 8. The 3rd stage larva of Nematodirus sp. a) The whole larva. b) The posterior part. Note the characteristic notched (fish-like) tail |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Discussion |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Domestic camels, Camelus dromedarius, are frequently parasitized with various helminth and protozoan species. Uniquely, they can withstand many important parasitic infections, so they appear less reliable to clinically-diagnosed helminthic infections that have potentially economic impact (Parsani et al., 2008; Borji et al., 2010). Endoparasitosis, in heavy infections, in camels often associated with a loss of the production particularly in long standing infections (Richard, 1989). Among those, the intestinal helminths greatly reduce the nutrients absorption causing tissue damage of the alimentary tract like retarded growth and emaciation (Faye, 1997; Rewatkar et al., 2009; Hamed 2018). Recently, gastrointestinal parasitism of camels got more interest as the animal feeding, breeding and diseases control have the ability to reduce the promising growth and production, thus, adversely affect the global camels husbandry (Radfar and Gowhari, 2013; El-Khabaz et al., 2019). The present work revealed that the overall prevalence of helminth eggs/protozoan oocysts, was 41.53% (260/626). Helminth eggs were detected in 28.11% of camels, protozoan oocysts were found in 5.59% and mixed infections of both were seen in 7.82% of animals. Fifteen species of both parasitic stages were recovered. Helminth eggs were those of various gastrointestinal nematodes, Trichuris sp., Moniezia sp. and Fasciola spp. Protozoan oocysts were those of coccidian parasites, Eimeria spp. (E. cameli, E. dromedarii and E. rajasthani) and Buxtonella sp. Among risk factors affecting the infection rates, significant difference, particularly for nematodes, was displayed relative to the age, and variable significant discrepancies for the recovered parasitic species (except for tapeworms) in relation to the seasons. No significant differences related to the sexes could be detected. Mixed infections with helminth eggs and protozoan oocysts, or even within the same class, were common. In Egypt, little previous literature with coproparasitological studies in domestic camels were done. Among those, Mahmoud et al. (2008), in the southern Egypt, detected that 41.08% of domestic camels were infected with nematode eggs. The recovered species were Nematodirus sp., Trichuris sp., Strongyloid spp. and Trichostrongylus spp. The later was the most predominant (20.0%). They revealed seasonal variations-based infection rates, but the effect of age and sex was not studied. Ahmed et al. (2013) reported a helminthic infection rate of 51.02% in camels slaughtered in Toukh, Qaluobia province. In Assiut, Hamed (2018) detected that 77.5% of camels had parasite eggs and protozoan oocysts. The recovered parasites were Eimeria spp. (55.0%), Trichostrongylus spp. (45.0%), Trichuris sp. (35.0%), Moniezia spp. (5.0%) and Ascaris spp. (5.0%). Moreover, she recorded both single and mixed infections. In Aswan province, El-Khabaz et al. (2019) reported a parasitic infection rate of 60.0% (72/120) in domestic camels coprologically examined. They added that, eighteen species of helminthes/protozoan parasites could be detected. The nematodes, trichostrongyles, were the most predominant (12.5%). In related African countries, Dia (2006) found only strongyle and Moniezia spp. eggs in Burkina Faso. In Tanzania, Swai et al. (2011) proved an infection rate of 62.7% with eleven species of helminth/protozoan parasites eggs/oocysts based on the coprological examination. Parallel to the current findings, they revealed a higher infection rates for Trichostrongylus spp. (27.7%) rather than Eimeria spp. (9.9%). Also, they detected significant differences among age grouping. Oppositely, they detected that female camels significantly haboured gastroinetstinal parasites eggs than males. In Ethiopia, Birhanu et al. (2014) revealed that the overall prevalence of gastrointestinal nematodes was 55.5%. The most common genera were strongyle eggs (48.7%) followed by Trichuris sp. (3.9%). Mixed infections were seen in 2.9%. In Nigeria, Mahmuda et al. (2014) reported that the overall prevalence of gastrointestinal parasites was 78.0%. The revealed parasites were strongyles eggs (62.96%), Strongyloides spp. (9.26%), Trichuris spp. (7.41%) and coccidian oocysts (8.33%). Muhomed et al. (2017) reported an overall prevalence of 79.0% on fecal examination in Somali state, Ethiopia. They revealed strongyle eggs (64.7%), Strongyloides spp. (22.3%), Trichuris spp. (12.2%), Paraphistomum spp. (1.7%), Fasciola spp. (2.1%) and Monezia spp. (8.4%). They found that, among risk factors, the age was significantly associated with the helminthic infection.
In Asian countries, Cirak et al. (2011) recorded that coprological examination revealed the presence of Trichostrongylus spp., Teladorsagia spp., Nematodirus spp., Trichuris spp., Capillaria spp., Anoplocephalidae, Dicrocoelium dendriticum, Eimeria cameli and E. rajasthani. In India, Chaturvedi et al. (2012) detected a lower prevalence, 28.5%. They detected strongyle, Trichuris and Strongyloides spp. eggs (71.92, 21.05 and 7.01%). In Iran, Radfar and Gowhari (2013) yielded that fecal examination revealed strongyle eggs and Eimeria oocysts in percentages of 64% and 24%, respectively. They revealed a significant association relative to the age of examined camels. Moreover, Al-Megrin (2015), in Saudi Arabia, detected that 59.6% of examined camels were infected with gastrointestinal parasites. Coprological examination recovered eggs of strongyles, Moniezia spp. and Stilesia spp. as well as E. cameli oocysts. She found the highest intestinal parasitic infections in summer. In Australia, Rashid et al. (2019) found that based on egg morphology, the overall prevalence of gastrointestinal nematodes in camels was 61.0% while that for strongyles was 53%. They detected Trichostrongylus spp., Haemonchus spp. and Camelostrongylus mentulatus the most predominant gastrointestinal nematodes. Furthermore, they recorded that the infection rate was higher in the winter rainfall zone rather than summer rainfall areas.
Currently, the infection rates were higher in male animals. Aged camels, more than 5 years, had the highest prevalence. Seasonally, it has been found that winter had the highest infection rate, followed by autumn, spring and summer. In Egypt, Ahmed et al. (2013) stated that ages of 1-5 years more susceptible to the parasitic infections, native breed had a higher infection rate than other breeds, and the high prevalence was significantly recorded in spring. Coinciding with the present findings, Birhanu et al. (2014) revealed infection rates of 69.05% in camels aged 5-10 years and 53.8% in those of more than 10 years. Moreover, they added that male animals were highly (64.7%) infected than females (55.04%). Similarly, Mahmuda et al. (2014) mentioned that lower-aged (90.0%) camels had a higher helminthic infection rate than old-aged (70.0%) animals, and the prevalence in male camels was 57.69%, while in females it was 42.31%.
It seemed that the tropical agro-climatic conditions, including the presence of wide deserts, high temperature and infrequent rainfall seasons, free-ranging management, the grazing behavior of dromedary camels as well as hygienic measures and anthelmintics treatment extremely contributed the higher prevalences (often more than 40%) of gastrointestinal parasites, particularly strongyles, in both African, including Egypt, and Asian countries. It is worth considering that the traditional habits of owners, that they prefer rearing in free-ranging system permit the existence of camels with various carrier and reservoir hosts, like cattle, sheep and goats, with higher possibilities to the transmission of parasitic stages (Abou El-Naga and Barghash, 2016; El-Khabaz et al., 2019). In fact, the discrepancy in infection rates might be probably associated the intensity of infection, number of adult parasites in the gastrointestinal tract, stage of the parasitic infection, the level of host immune response or the improper animal management (Sharrif et al., 1996; El-Khabaz et al., 2019). Interestingly, during the long trips done by camels as well as the unexpected and drastically alterations in climatic conditions in various seasons, the maintenance and development of parasitic larval stages are greatly variant, thus, explaining the irregular occurrence of parasitic helminth eggs and/or protozoan cysts among different districts, even of the same country (Mahmoud et al., 2008).
Regarding gastrointestinal (GIT) nematodes, the present work revealed, based on coprological examination and fecal culture, the presence of the third larval stages (L3) of 8 trichostrongylids; Trichostrongylus axei, Tr. colubriformis, Chabertia ovina, Ostertagia ostertagi, Haemonchus sp., Oesophagostomum sp., Bunostomum sp. and Nematodirus sp. For an accurate larval identification, both the anterior (head) and posterior (tail) extremities are useful taxonomic features. Meanwhile, the morphology of L3 tail could be used as a unique criterion to differentiate larvae of GIT nematodes, particularly trichostrongyles (McMurtry et al., 2000). The length of L3 might be varied as a result to the host immune mechanism that may alter the size of both eggs and larvae (Hong and Timms, 1986; Douch et al., 1988; McMurtry et al., 2000).
It has been found that the number of tubercles on the tail of the L3 can be used to identify and differentiate Trichostrongylus spp. Accordingly, L3 with a simple tail is recognized as Tr. axei, and that with 2-3 tubercles is defined Tr. colubriformis (Hollo et al., 1970; Anonymous 1986). Herein, Trichostrongylus axei and Tr. colubriformis were revealed. The length of both the larva and its STE may vary according to the amount of moisture in the culture medium, so, an expertise of such instance is requested to maintain the whole measurements consistent throughout (Rossanigo and Gruner, 1996; van Wyk et al., 2004).
L3 of Chabertia ovina is characterized by the long whole length, square-shaped head and rectangular-shaped intestinal cells (> 16) with the tail filament approximately 25-30% of STE (van Wyk and Mayhew, 2013). C. ovina L3 was yielded in this study. Ostertagia ostertagi is identified based on the rounded-shaped head, 16 triangular intestinal cells and a blunt tail tip without a filament. The current study revealed the occurrence of O. ostertagi. Haemonchus sp. L3 were obtained from feces of examined camels. Characteristically, it has bullet head, zigzag-shaped intestinal cells (Veglia, 1916) and a pointed tail. Meanwhile, the fecal culture media revealed the presence of Oesophagostomum sp. L3. It had a characteristic long filament (van Wyk and Mayhew, 2013). The same authors reported that L3 of Bunostomum sp. has bullet head and a long filament. Accordingly, Bunostomum sp. was obtained in this work. Due to being the fact that Nematodirus sp. eggs need up to 14 days for hatching, fungal overgrowth commonly occurs. Therefore, it was interesting to obtain Nematodirus sp. in the present investigation (van Wyk and Mayhew, 2013). Uniquely, L3 of Nematodirus sp. is very long and the terminal end of the tail is notched (fish-like).
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conclusion |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Further questionnaires and studies, including molecular approach, are required to screen various helminth and protozoan parasites in camels, both native and imported, in Egypt.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Acknowledgments |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Authors deeply thank all veterinarians and workers, who facilitate fecal samples collection.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conflict of Interests |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Authors declare that there is no conflict of interest. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
References |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abou El-Naga, T.R., Barghash, S.M., 2016. Blood parasites in camels (Camelus dromedarius) in Northern West Coast of Egypt. Journal of Bacteriology and Parasitology 7, 1-7. Al-Megrin, W.A., 2015. Prevalence rate of intestinal parasites in camels in Riyadh, Saudi Arabia. International Journal of Zoological Research 11, 65-70. Ahmed, N.E., El-Akabway, L.M., Ramadan, M.Y., Abd El-Gawad, S.M., 2013. Detection and identification of some helminth parasites affecting camels. Egyptian Journal of Veterinary Science 44, 81-92. Anonymous, 1986. Manual of Veterinary Parasitological Laboratory Techniques. Ministry of Agriculture Fisheries and Food, Technical Bulletin No. 18, HMSO, London, 160 pp. Banaja, A.A., Ghadour, A.M., 1994. A review of parasites of camels (Camelus dromedarius) in Saudi Arabia. Journal of King Abdulaziz University Science 6, 75-86. Birhanu, T., Alebie, A., Giro, B., Chanie, M., 2014. Prevalence of gastrointestinal nematodes of camel slaughtered at Akaki abattoir, Addis Ababa, Ethiopia. Acta Parasitologica Globalis 5, 177-182. Borji, H., Razmi, G., Movassaghi, A.R., Naghibi, A.G., Maleki, M., 2010. A study on gastrointestinal helminths of camels in Mashhad abattoir Iran. Iran Journal of Veterinary Research 11, 174-179. Chaturvedi, V., Tanwar, R.K., Fakhruddin, Chahar, A., Singh, A.P., 2012. Prevalence of gastrointestinal helminths in camels of Bikaner and Jaisalmer district of Rajasthan. Veterinary Practitioner 13, 82-83. Cirak, V.Y., Senlik, B., Gulegen, E., 2011. Gastrointestinal parasites of camels (Camelus dromedarius) from Turkey and efficacy of doramectin against trichostrongyles. Journal of Camel Practice and Research 18, 283-285. Dia, M.L., 2006. Parasites of the camel in Burkina Faso. Tropical Animal Health and Production 38, 17-21. Douch, P.G., Harrison, G.B.L., Buchanan, L., Greer, K.S., 1988. Relationship of nematode cholinesterase activity and nematode burdens to the development of resistance to Trichostrongyle infections in sheep. Veterinary Parasitology 27, 291-308. Dubey, J.P., Schuster, R.K., 2018. A review of coccidiosis in Old World camels. Veterinary Parasitology 262, 75-83. El-Dakhly, Kh. M., El-Nahass, E., Uni, S., Tuji, H., Sakai, H., Yanai, T., 2012. Levels of infection of gastric nematodes in a flock of great cormorants (Phalacrocorax carbo) from Lake Biwa, Japan. Journal of Helminthology 86, 54-63. Elfadaly, S., 2016. Camel Diseases. GF‐TADs Sub-regional conference on camel diseases, Abu Dhbi – United Arab Emirates. El-Khabaz, K.A.S., Abdel-Hakeem, S.S., Arfa, M.I., 2019. Protozoan and helminthes parasites endorsed by imported camels (Camel dromedaries) to Egypt. Journal of Parasitic Diseases 43, 607-615. Faye, B., 1997. Guide d’elevage du dromedaire. Edition CIRAD-EMVT, Montpellier, p. 126. Hamed, M.I., 2018. Ivermectin resistance in intestinal parasites of camels in a private farm at Assiut, Egypt. Comparative Clinical Pathology 27, 1221-1226. Hollo, F., Rovo, J.T., Hidvegi, Z., 1970. A recent case of human Trichostrongylus infection in Hungary. Parasitologia Hungarica 3, 159-168. Hong, C., Timms, B.J., 1986. Variation in size of Ostertagia circumcincta, a nematode parasite of sheep, induced experimentally and during preparation and preservation. Systematic Parasitology 9, 39-42. Khatami, A.K., 2016. Performance of production and trade of camel products in some Middle East countries. African Journal of Agricultural Economics and Rural Developments 4, 393-399. Mahmoud, M.A., Amin, M.M., Youssef, R.R., El-Kattan, A., Goda, A.S.A., Abou El-Naga, T.R., 2008. Studies on some endoparasites of camels in the southern area of Egypt. Suez Canal Veterinary Medicine Journal 13, 81-92. Mahmuda, A., Mohammed, A.A., Alayande, M.O., Habila, Y.I., Lawal, M.D., Usman, M., Raji, A.A., Saidu, B., Yahaya, M.S., Suleiman, N., 2014. Prevalence and distribution of gastrointestinal parasites of working camels in Sokoto metropolis. Veterinary World 7, 108-112. Markell, E.K., John, D.T., Krotoski, W.A., 1999. Markel and Voge's Medical Parasitology. 8th edn. W.B. Saunders. McMurtry, L.W., Donaghy, M.J., Vlassoff, A., Douch, P.G.C., 2000. Distinguishing morphological features of the third larval stage of ovine Trichostrongylus spp. Veterinary Parasitology 90, 73-81. Mouldi, S.M., Olfa, B., Najet, Z., Imed, S., Touhami, K., 2015. Effects of two anthelmintics on gastrointestinal infestation by parasitic worms in camels. Emirates Journal of Food and Agriculture 27, 390-395. Muhomed, M., Sibhat, B., Kemal, J., 2017. Camel gastrointestinal helminths in selected districts of Somali regional state, eastern Ethiopia. Livestock Research for Rural Development. Volume 29, Article #49. http://www.lrrd.org/lrrd29/3/jela29049.html Parsani, H.R., Singh, V., Momin, R.R., 2008. Common parasitic diseases of camel. Veterinary World 1, 317-318. Radfar, M.H., Gowhari, M.A., 2013. Common gastrointestinal parasites of indigenous camels (Camelus dromedarius) with traditional husbandry management (free-ranging system) in central deserts of Iran. Journal of Parasitic Diseases 37, 225-230. Rashid, M.H., Stevenson, M.A., Vaughan, J.L., Saeed, M.A., Campbell, A.J., Beveridge, I., Abdul Jabbar, 2019. Epidemiology of gastrointestinal nematodes of alpacas in Australia: II. A longitudinal study. Parasitology Research 118, 901-911. Rewatkar, S.G., Deshmukh, S.S., Deshkar, S.K., Maske, D.K., Jumde, P.D., Bhangale, G.N., 2009. Gastrointestinal helminths in migratory Camel. Veterinary World 2, 258. Richard, D., 1989. Manuel des maladies du dromedaire. IEMVT, AlfortSchillhorn Van Veen T.W.C.,1986. Coccidiosis in ruminants'': a review. The Compendium on Continuing Education for the Practicing Veterinarian 8, F52–F58. Rossanigo, C.E., Gruner, L., 1996. The length of strongylid nematode infective larvae as a reflection of developmental conditions in faeces and consequence on their viability. Parasitology Research 82, 304-311. Saber, A.S., 1998. The Camel in Ancient Egypt. Proceedings of the Third Annual Meeting for Animal Production Under Arid Conditions, Vol. 1, 208-215. Sharrif, L., Al-Qudah, K., Al-Ani, F.K., 1996. Prevalence of gastrointestinal parasites in camels in Jordan. Journal of Camel Practice and Research 5, 1-4. Soulsby, E.J.L., 1982. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th edn. London, UK. Swai, E.S., Moshy, W., Mshanga, D., Lutatina, J., Bwanga, S., 2011. Intestinal parasitic infections of camels in the agro and pastoral areas of northern Tanzania. Livestock Research for Rural Development Volume 23, Article #115. http://www.lrrd.org/lrrd23/5/swai23115.htm van Wyk, J.A., Cabaret, J., Michael, L.M., 2004. Morphological identification of nematodes of small ruminants and cattle simplified. Veterinary Parasitology 119, 277-306. van Wyk, J.A., Mayhew, E., 2013. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort Journal of Veterinary Research 80, Art. #539, 14 pages. http://dx.doi.org/10.4102/ojvr.v80i1.539 Veglia, F., 1916. The anatomy and life-history of Haemonchus contortus (Rud.). Union of South Africa. Dept. of Agriculture (Pretoria: Government Printer and Stationery Office, 1916) 500 pp. Wernery, U., Kadden, O.R., 2002. Infection diseases in camelids. 2nd edn. Blackwell, Berlin. Zajac, A.M., Conboy, G.A., 2006. Veterinary Clinical Parasitology, 7th edn. Blackwell, Ames. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||