|
|
Journal of Advanced Veterinary Research Volume 10, Issue 1, 2020, Pages: 41-48 www.advetresearch.com |
|
|
Gross, Light and Scanning Electron Microscopic Studies on the Ampulla Ductus Deferentis of Dromedary Camel with Special Reference to its Seasonal Variations |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Abdelmohaimen M.M. Saleh1*, Ramy K.A. Sayed2, Hazem Hamoda3 |
||||||||||||||||||||||||||||||||||
|
1Department of Anatomy, Histology and Embryology, Faculty of Veterinary Medicine, Assiut University, 71515 Assiut, Egypt. 2Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Sohag University, 82524 Sohag, Egypt. 3Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Aswan University, 81528 Aswan, Egypt. |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Received: 17 December 2019; Accepted: 7 January 2020 (*: Corresponding author: abdel_mohaimen@aun.edu.eg) |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Abstract |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
The morphological structure and morphometrical features of the ampulla ductus deferens of the adult camels were studied by light and scanning electron microscopy to get better understand with its seasonal variations. The wall of ampulla was composed of mucosa, submucosa, muscularis and serosa or adventitia. It was lined by pseudostratified columnar epithelium containing intraepithelial glands. The lamina propria and tunica submucosa formed together the thickest part of the ampullary wall. The ampullary glands were branched tubulo-alveolar, with diverticulae-like appearance and occupied mostly the lamina propria- submucosa. Each gland consisted of peripheral wide and central narrow alveoli that were lined by simple low columnar or cuboidal epithelium and mostly contained spermatozoa and secretory materials. The gland opened in the ampullary lumen by short tubule, which was lined by pseudostratified columnar epithelium. Histochemically, the epithelial cells reacted positively to Alcian blue, PAS and sudan black stains and negatively to the Best's carmine stain, indicating the presence of the acid, neutral glycoprotein and fatty droplet, as well as absence of the glycogen. Morphometrically, the height of the luminal and glandular epithelia, thickness of the lamina propria- submucosa and ratio of the glandular to the connective tissue showed seasonal variations. The height of the luminal and glandular epithelia reached their maximum values in winter and decreased gradually throughout spring and recorded the lowest values in summer. Scanning electron microscopy revealed various shaped openings in the luminal surface of the ampulla. The cells apices were studded with short microvilli and many secretory granules or vesicles. The ampullary glands appeared as a network of diverticulae-like structure, which occupied mostly the lamina propria-submuosa. The cells apices of the glandular epithelium were stereo-ciliated, microvilliated cells or showed central bleb-like protrusion surrounded by thin long microvilli. The glandular alveoli contained spermatozoa and secretory materials. In conclusion, the camel ampulla ductus deferentis performs a storage function in addition to its secretory one, where both are subjecting to seasonal variations. |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Keywords: Ampullary gland, Ductus deferens, Morphology, SEM, Spermatozoa |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Introduction |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
The accessory genital glands have an essential role in the reproductive process, as they are the main contributors to the seminal volume fluid, as their secretions constitute about 60-90% of the total volume of semen (Chughtai et al., 2005; Dukes, 2005). These secretions provide environment of suitable substrates for seminal motility and fertility, and for transmitting sperm to the female (McDonald, 1980; Banks, 1993; Davies Morel, 2003). The morphological features of the accessory sex glands show wide differences among various mammalian species (Wrobel and Dellmann, 1993), including one-humped camel (Ali et al., 1978; Thomson and Marker, 2006). The vas deferens or ductus deferens of most mammalian species differentiates anatomically into two portions; proximal ductal and distal ampullary (Setchell et al., 1994). The ampullary portion is known in mammals as ampulla ductus deferentis, which is further termed as ampullary gland as it is primarily a secretory organ (Riva et al., 1989). The ductus deferens plays an important role in the viability of sperm, as well as its protection against reactive oxygen species and proteases during storage (Hinton et al., 1996). Furthermore, the ampulla of the ductus deferens has been confirmed to plat an active role in sperm nourishment and maturation (Bergerson et al., 1994). Many previous studies were performed to investigate the histological structure and scanning electron microscopical features of the ampulla ductus deferens in different animal species including man (Riva et al. 1982; Nistal et al., 1992), dogs (Murakami et al., 1986), golden hamster (Chow, 1988) and donkeys (Abou-Elhamd, 2012, 2013). In camels, some studies were carried out to demonstrate the histological and histochemical features of the ductus deferentis (Ali et al., 1978; Mosallam, 1981; Goswami et al., 1990); however, scanning electron microscopical features of this organ in camels have not been investigated. Thus, this study was designed to highlight the morphological and morphometrical features of the ampulla ductus deferentis of dromedary camel by light and scanning electron microscopy, and also to get better understand with its seasonal variations. |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Materials and methods |
||||||||||||||||||||||||||||||||||
|
This study was carried out on ampulla ductus deferentis of twenty-five sexually mature and apparently healthy camels (Camelus dromedarius) obtained from Bany-Ady slaughterhouse at Assiut governorate, Egypt throughout the year (representing winter, spring, summer and autumn seasons). All methods were performed according to the ethical regulations and relevant guidelines of the Faculty of Veterinary Medicine, Assiut University, Egypt. The ampulla was examined grossly while it was intact for its topographical relations. Moreover, the length and diameter of the ampulla were measured by using digital caliper. Light Microscop examinationSamples (1×1 x 0.5 cm) from the ampulla were dissected freshly from different parts and fixed freshly in neutral buffered formaldehyde, Bouin´s fluid (for routine histological and morphometrical examination), Carnoy´s fixative (for carbohydrates histochemistry) and cold formol-calcium (for lipids). The fixed samples were processed for paraffin sectioning through dehydration in ethyl alcohol, clearing in methyl benzoate and embedding in paraffin wax. Sections (4-7 µm thick) were cut and stained by haematoxylin and eosin (Harris, 1900) for general histological examination, Periodic acid-Schiff (PAS, McManus, 1946) and Alcian blue (Steedman, 1950) for carbohydrates histochemistry, Best´s carmine for detection of glycogen (Best, 1906) and Sudan black (Lison, 1960) for demonstration of lipids. Staining was prepared according to Bancroft’s theory and practice of histological techniques (Bancroft et al., 2013). Stained sections were examined and photographed using a Leica microscope (Germany). Scanning electron microscopical studies Small specimens of the ampulla were washed by 0.1M sodium phosphate buffer and immersed in a mixture of 2.5% glutaraldehyde and 2.5% paraformaldehyde in 0.1M sodium- phosphate buffer at pH 7.2 for fixation at 4 ºC. The samples were then post-fixed in 1% osmic acid in 0.1M Na-cacodylate buffer for 2 h at room temperature, dehydrated in alcohol followed by amyl acetate. Specimens were dried by the critical point drying using liquid nitrogen and mounted on specimen’s tubes. The samples were sputter-coated with gold, viewed and photographed with JEOL JSM-5400 LV scanning electron microscope at Electron microscopy unit, Assiut University, Egypt. Morphometrical examination Some morphometric aspects including height of lamina epithelialis, height of glandular epithelium, thickness of lamina propria –submucosa and thickness of tunica muscularis, as well as interstitial connective tissue / glandular tissue ratio of the lamina propria-submucosa were performed using Leica Q 500 MC Image analyzer and scanning electron microscope. Statistical analysis For statistical analysis, a one-way ANOVA with a Tukey's post hoc test was performed to compare the morphometrical differences between groups. The data were presented as Mean±SD of 6 animals per group. The results were considered statistically significant when P-value ˂ 0.05. |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Results |
||||||||||||||||||||||||||||||||||
|
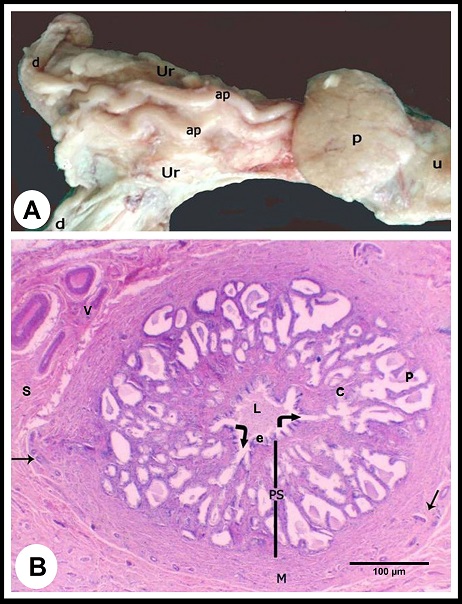
Macroscopic observations The ampulla ductus deferens of camel is located in the pelvic cavity between the two layers of the urogenital fold dorsal to the urinary bladder and medial to the ureter (Fig. 1A). Within the urogenital fold, the ampulla runs tortuous, and its terminal part is retroperitoneal, traverses the prostate to open in the pelvic urethra. The ampulla measured 18.0± 2.0 cm in length and 6.0±2.0 mm in diameter.
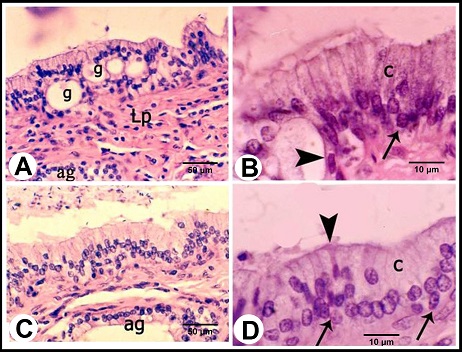
Fig. 1. (A):The ampulla ductus deferentis (ap) runs tortuous within the layers of the urogenital fold (ur). (d) ductus deferens, (p) prostate gland, (u) pelvic urethra. (B): Cross section in the wall of the ampulla ductus deferentis showing the lining mucosa that was slightly folded surrounding the central lumen (L). The ampullary glands (Curved Arrows) are branched tubuloalveaolar type, consisted of narrow central alveoli (c) and wide peripheral ones (P). (e) lamina epithelialis, (PS) lamina propria-submucosa, (M) Tunica muscularis, (V) the vascular layer of the tunica muscularis, (Arrows) the muscle bundle derived from the inner layer of the tunica muscularis toward the lamina propria-submucos, (S) connective tissue of the serosa, H&E stain. Histological examination The wall of ampulla ductus deferentis consists of mucosa, submucosa, muscular coat, and serosa or adventia (Fig. 1B). The mucosa is composed of lamina epithelialis and lamina propria. The former is formed of pseudostratified columnar epithelium with brush borders and contains intraepithelial glands. It is formed mainly of two types of cells; columnar that is the principle cell type and basal one. The columnar cells are tall and characterized by granulated acidophilic cytoplasm and variable-shaped nuclei (round, oval or elongated). They showed seasonal variations; from the beginning of October, these cells increased in height and their cytoplasm became granulated. They reached to the maximum height in winter (Figs. 2A, B), then decreased again throughout the spring to record its minimum height in summer where most of the cells became non-granulated (Figs. 2C, D). The basal cells are oval or round in shape wedged between the basal portions of the principle ones, and characterized by acidophilic non-granulated cytoplasm with their nuclei stained darker. Some rod-shaped cells were singly scattered among the columnar cells. They were small cells differentiated by their deeply stained acidophilic cytoplasm and oval or elongated deeply stained nuclei (Fig. 2D).
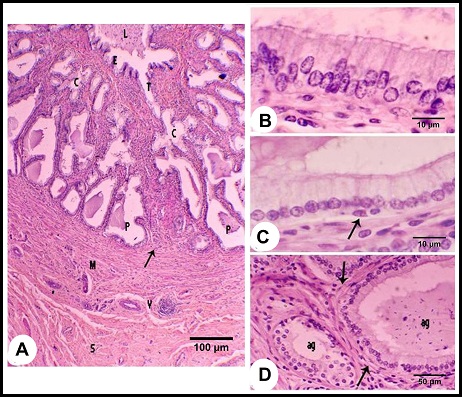
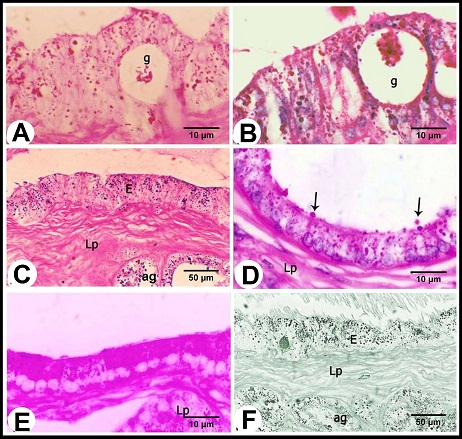
Fig. 2. (A & B): During the winter season, lamina epithelialis shows intraepithelial glands (g). The principle cell shows granulated acidophilic cytoplasm (c). The intraepithelial glands are lined by flattened epithelium (Arrow head). (Lp) lamina propria, (ag) ampullary glands epithelium, (Arrow) the basal cells, H&E stain. (C & D): During the summer season, lamina epithelialis is consisted of pseudostratified columnar epithelium without intraepithelial glands. The cytoplasmic granulation of the principle columnar cells (c) is weak or absent. (Arrow head) rod-shaped columnar cells, (Arrows) basal cells, (Lp) lamina propria, (ag) ampullary glands epithelium, H&E stain. The intraepithelial glands were more pronounced during the winter and early spring. They were simple tubular glands found in the lamina epithelialis not reached to the lamina propria. They were lined by simple cuboidal or flattened cells. These cells were characterized by an acidophilic non-granulated cytoplasm and small oval or flattened nuclei. Their lumina contained acidophilic materials (Figs. 2A, B). The lamina propria and submucosa formed together the thickest portion of the wall of the ampulla, which was occupied mostly with the ampullary glands. The interstitial tissue formed of dense connective tissue containing collagenous, elastic and smooth muscle fibers. The Smooth muscle fibers arose firstly as bundles from the inner layer of the tunica muscularis and distributed between the glands forming a thin muscular layers around the secretory portions in addition to singly scattered smooth muscle fibers within the connective tissue. Small blood vessels and capillaries as well as fibroblasts were also observed. (Figs. 1B, 3A). The ampullary glands were of the branched tubulo-alveolar type, which mostly had diverticulae- like appearance, increased in diameter towards the periphery (Figs. 1B, 3A). Each gland was composed of wide peripheral and narrow central alveoli and opened in the central lumen by short tubule. This tubule lined by pseudostratified columnar epithelium with brush border. The alveoli were lined by simple low columnar or cuboidal epithelium, which was formed mainly of principle cells and very few basal ones. The principle cells were low columnar in the central alveoli and cuboidal in the peripheral ones, both were characterized by brush borders and distinct cell boundaries. They possessed foamy non-granulated acidophilic cytoplasm and round or oval vesicular nuclei. The basal cells were very few and were oval or flattened in shape, their cytoplasm was similar to that of principle cells but their nuclei were stained darker. The lumina of alveoli contained variable amounts of secretory substance and spermatozoa (Figs. 3B, C).
Fig. 3. (A): The ampullary glands are consisted of narrow central alveoli (c) and wide peripheral ones (P), and opened in the central lumen by short tubule (T). (E) lamina epithelialis, (M) Tunica muscularis, (V) the vascular layer of the tunica muscularis, (Arrow) the muscle bundle derived from the inner layer of the tunica muscularis toward the lamina propria-submucos, (S) connective tissue of the serosa, H&E stain. (B & C): The tubules of the ampullary gland are lined by pseudostratified columnar epithelium with brush border, while the alveoli line by low columnar epithelium with few basal cells (Arrow), H&E stain. (D): The glandular alveoli are surrounded by a thin layer (Arrows) (ag), H&E stain. The tunica muscularis was rich in the intermuscular connective tissue, formed of variably arranged smooth muscle bundles. Its outer layer formed a vascular layer. It was rich in the blood vessels (arterioles, venules and blood capillaries) (Figs. 1B, 3A, D). The tunica serosa covered the ampulla within the genital fold, while the rest was invested with tunica adventitia. The tunica serosa was formed of flat mesothelial cells and a thick submesothelial connective tissue layer. The latter as well as the adventitia were composed mainly of collagenous fibers adipose tissue, fibroblasts and blood vessels of variable calibers (Figs. 1B, 3A). Histochemical analysis The lamina epithelialis and glandular epithelium of the secretory tubules and central alveoli of the ampullary glands reacted moderately positive with PAS stain. These reactions were represented by positive granules of variable sizes distributed in the cytoplasm of the principle cells and few fine granules in the basal cells. The lining epithelium of the peripheral alveoli reacted strongly positive with the PAS stain in the form of diffuse homogenous positive substance filling the cytoplasm of the principal cells. Some glandular alveoli and some principle cells were either weakly or negatively reacted. The seasonal variation in the in the PAS stain were not clear (Fig. 4A). Alcian blue staining showed fine granular reaction with clear background in the lamina epithelialis and glandular epithelium, while other layers of the ampulla ductus deferentis were alcian blue negative. Combination of alcian blue /PAS staining showed a mixture of the granular reaction in the lamina epithelialis and the glandular epithelium of the central alveoli. In the peripheral alveoli it showed strong homogenous PAS and few alcian blue granules. The lining epithelium of intraepithelial glands also reacted positively with the PAS and alcian blue stains and their lumina contained mixture positive materials (Figs. 4B-E). Furthermore, the cells of the lamina epithelialis and the glandular epithelium showed fine sudanophilic granules (Fig. 4F).
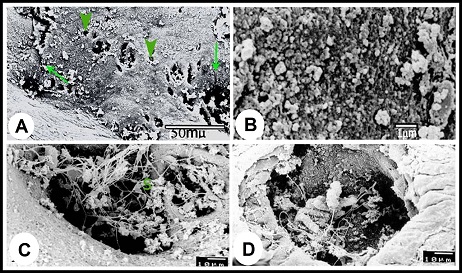
Fig. 4. (A & B): during the winter season, the lamina epithelialis and intraepithelial glands (g) contain PAS-positive granules and a mixture of Alcian blue- and PAS-positive granules; (g) intraepithelial glands, PAS & Alcian /PAS stain. (C): While during the summer, the lamina epithelialis (E) and the lining epithelium of the central alveoli (ag) contain a mixture of alcian blue- and PAS-positive granules. (Lp) lamina propria, Alcian /PAS stain. (D & E): The central alveoli showing granular reaction with the Alcian blue /PAS stain, while the peripheral alveoli showing strong homgenous PAS-positive reaction and few Alcian blue-positive granules. (Arrows) the bleb-like projections are PAS-positive, Heamatoxylin -Alcian /PAS stain. (F): The tunica mucosa showing positive sudanophilic granules in both the lamina epithelialis (E) and glandular epithelium (ag). (Lp) lamina propria, Sudan black stain. Scanning electron microscopical examination Scanning electron microscopy of lamina epithelialis showed that the luminal surface of the ampulla ductus deferentis had low irregular folds and the openings of the ampullary glands. These openings appeared in variable sizes and shapes (irregular, rounded, oval, of slit-like) containing secretory materials. Also there were many small round openings for the intraepithelial glands distributed among that of the ampullary glands (Fig. 5A). The higher SEM magnification showed that the lamina epithelialis was made up of irregular polygonal cells with indistinct cell boundaries carrying microvilli and secretory granules and microvilli (Fig. 5B). Some spermatozoa were demonstrated in contact with the surface epithelium (Figs. 5C, D).
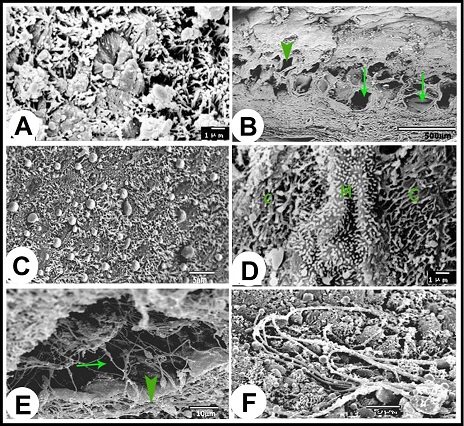
Fig. 5. (A): Scanning electron micrograph showing the luminal surface of the ampulla ductus deferetis; (Arrows) large different shape openings for the ampullary glands, (Arrow heads) small round openings for the intraepithelial glands. (B): The apices of the lamina epithelialis cells carried microvilli and secretory granules. (C & D): The openings of the ampullary glands were different shapes usually contain sperms (S) and secretory materials. The lamina propria-submucosa was mostly occupied by the alveoli of the ampullary glands, which appeared in the scanning electron micrographs as diverticules. The diameter of these diverticulae ranged from 40 to 380 µm. The higher SEM magnification showed that the cells surfaces of the glandular epithelium appeared different from place to other. In the glandular tubules it was stereociliated, where apices of the cells were studded with dense sterocilla (Fig. 6A). The alveoli of the ampullary glands appeared in form of network of diverticules where the narrow alveoli found toward the lumen and the wide ones demonstrated towards the periphery (Fig. 6B). In these glandular alveoli, the apices of most cells showed large central bleb-like protrusions and many small ones distributed on the surface of the cells in addition to long thin microvilli concentrated around the circumference of the cells apices (Fig. 6C). In some alveoli, the cells were studded with long thin microvilli interrupted with other cells studded with short microvilli arranged in lines with clear interval (Fig. 6D). The glandular alveoli were filled with spermatozoa and secretory materials. The spermatozoa were arranged in layers attached to the epithelium or free in the centers of the lumina (Fig. 6E). As shown in the lamina epithelialis, some spermatozoa appeared indenting the cells apices of the glandular epithelium (Fig. 6F).
Fig. 6. (A): The lining epithelium of the tubular parts of the ampullary glands is studded with sterocilia. (B): In the longitudinal section of the wall of the ampulla, the alveoli of the ampullary glands appeared in form of network of diverticules; the narrow alveoli (Arrow head) present toward the lumen and the wide ones (Arrows) present towards the periphery. (C): The apices of majority lining cells in the glandular alveoli carried large central bleb-like structure surrounded by long and thin microvilli in the circumference of the cells. (D): The epithelium lining the glandular alveoli consisted of three types of cells; cells carried long and thin microvilli (C), cells carried short and thick microvilli arranged in rows (M). (E): Inside the alveoli, the spermatozoa arragenged in layers (Arrow head) in addition to many free ones in the center of the diverticules (Arrow). (F): Some spermatozoa appeared indenting the cells apices of the glandular epithelium or presented deeply between them. Morphometrical analysis As shown in Table 1, the height of the lamina epithelialis and glandular epithelium as well as the thickness of the lamina propria-submucosa revealed significant seasonal variations, where the maximum values (Except for the thickness of the lamina propria-submucosa) were reported in winter and decreased gradually throughout the spring and reached the lowest ones in the summer. The ratio of interstitial connective tissue to glandular tissue in the lamina propria-submucosa showed also significant alterations among the seasons of the year. The lowest proportion was detected in the winter and gradually increased to reach the highest ratio in the summer and autumn. The thickness of the tunica muscularis however demonstrated insignificant seasonal variations. Table 1. Morphometrical analysis (µm) of camel ampulla ductus deferentis throughout the year.
Date were presented as Mean±SD. In each row, a p<.05, b p<.01 and c p<.001 vs. Winter; d p<.01 and e p<.001 vs. Spring; f p<.05, g p<.01 and h p<.001 vs. Summer. |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Discussion |
||||||||||||||||||||||||||||||||||
|
The accessory sex glands of the male genital system play a critical role in the reproductive process (Chughtai et al., 2005), and their morphology showing wide variations among different animal species (Thomson and Marker, 2006). Among these glands, the ampulla of ductus deferens is a glandular enlargement at the terminal part of the ductus deferens near the urethtra (Frandson et al., 2009; Khalaf and Merhish, 2010; Mai, 2014), where it narrows and passes ventrally under the prostate body to empty their contents into the colliculus seminalis (Mahmud et al., 2016). In camel, the ampulla ductus deferens was lined by pseudostratified columnar epithelium containing intraepithelial glands and showing seasonal variations. The height of the lining epithelium recorded its maximum value in the winter and decrease gradually to reach the minimum in the summer. The cytoplasm of the epithelial cells was granulated in the winter and non-granulated in the summer. However, Mosallam (1981) reported in the same animal species that the height of lining epithelium and the cytoplasmic granulation reached their maximum in spring season and the minimum during the summer. These cytoplasmic granules were previously proposed as a correspondence to the sub-cellular fractions; the lysosomes, in which the enzymes were detected (de Duve et al., 1962). The presence of cytoplasmic granulations is an indicator for cell activity increase in winter season than other ones. The lamina epithelialis was formed of columnar and basal cell types, with few small rod-shaped cells scattered among columnar cells. These small rod-shaped cells were previously suggested as a marker for old or exhausted columnar secretory cells (Nistal et al., 1992), where these cells have undergone mitochondrial hyperplasia and loss of organelles involved in glycoprotein synthesis. Furthermore, Paniagua et al. (1982) suggested involvement of these cells in the seminal plasma acidification or transport of electrolytes, hydrogen ions and water across the mucosa. The basal cells were proposed to be undifferentiated cells that are capable of differentiation into columnar cells, replacing the dead sloughing cells (Paniagua et al., 1982; Riva et al., 1982), and some of these cells showed PAS and alcian blue positive granules, which indicates their secretory activities. The intraepithelial glands as detected in this study were more pronounced during the winter season and became less developed during the other seasons. They were lined by simple cuboidal or flattened epithelium and their lumina contained acidophilic materials. Their lining cells and lumina contained mixture of Alcian blue and PAS positive granules indicating their secretory nature. Such these intraepithelial glands were not previously described in the ampulla ductus deferentis of the domestic animals, as it has been only recorded in the body segment of camel epididymis during the winter season (Singh and Bharadwaj, 1980; Saleh, 2002). The appearance of these glands during the mating season may indicate their role in seminal fluid secretions. The lamina propria and submucosa of the ampulla formed the thickest layer that was occupied mostly by the ampullary glands, a similar finding reported by Ali et al. (1978) and Goswami et al. (1990) in the same animal. The interstitial tissue of lamina propria-submucosa was composed of dense connective tissue containing elastic, reticular, collagenous and smooth muscle fibers. The elastic fibers formed a subepithelial network, where the girdle-like arrangement of these fibers provides the ampulla with a great elasticity to counteract the mechanical stress exerted by the luminal contents (secretory materials and spermatozoa) from one side and the powerful muscular contraction from the opposite side (Mosallam, 1981; Liebich, 1990). Moreover, the smooth muscle fibers revealed their continuation from the muscular layer and depicted their distribution around the glandular diverticulae till reaching the subepithelial layer. The presence of these muscular fibers in such manner probably act as supporting element of fully packed ampullary glands and their contraction may help in evacuating the secretory substances and stored sperms during the ejaculation. The ratio of the interstitial connective tissue to the glandular tissue revealed seasonal variations, where the lowest ratio was detected in the winter and the highest value was reported in the summer. The epithelial height reached its maximum in the winter and its minimum in the summer. Consequently, these findings may be related to the fluctuation of the androgen level in the camel during different seasons; being the highest in the winter and early spring and the lowest in fall of summer (Derar et al., 2005). Mosallam (1981), however, reported that the glandular epithelium reached its maximal activity during the spring and lowest activity during the summer. The ampullary glands of the camel were either branched tubulo-alveolar type (Goswami et al., 1990) or branched tubular (Ali et al., 1978). The ampullary glands detected in this study were of branched tubulo-alveolar variety and mostly had diverticulae-like appearance that increased in diameter towards the periphery, similar to those reported by Mohammed and Doohi (2017) in indigenous gazelle and by Wrobel and Dellmann (1993) in domestic animals, however, the tubular type was reported only in elephants (Short et al., 1967) and in rams (Perera, 1974). The peripheral alveoli were large with wide lumina, and were lined with cuboidal epithelium with brush border and very few basal cells, while the central alveoli were smaller in size with narrower lumina and lined with low columnar epithelium with brush border and few basal cells. These results were consistent with that recorded by Goswami et al. (1990) and inconsistent to that described by Ali et al. (1978) who stated that the peripheral alveoli of the camel ampulla were lined by tall columnar epithelium and the central ones lined by low columnar epithelium. The scanning electron microscopy revealed that the glandular alveoli were filled with spermatozoa and secretory materials. The apices of the lining cells appeared in different pictures: The first one in the tubules, the cells apices studded with dense and long sterocilla that might help in the movement of the sperms and secretion in and out of the glandular alveoli. The second in the alveoli, the apices of most cells showed large central bleb-like protrusions and many small ones and surrounding by thin long microvilli concentrated around the circumference of the cells apices. The bleb-like protrusions at the apical borders of the principal cells might indicate an apocrine mode of secretion, while the other with small bleb like protrusions might show a merocrine mode of secretion. These results go with that of Chow and Pang (1989) who suggested that the ampulla ductus deferentis discharged the fatty materials by apocrine mode of secretion, while the proteinaceous was exported by merocrine mode. The third picture present in some alveoli, where their cells were studded with thin long microvilli interrupted by others with short microvilli arranged in lines with clear interval. The microvilli may increase the surface area for water reabsorption and luminal fluids concentration. The presence of a large amount of the spermatozoa in tubular and alveolar lumina of the camel ampulla indicates that it might store spermatozoa for a time before ejaculation (Perera, 1974; Ali et al., 1978). In addition, it has been reported that the sperms complete their maturation within the ampulla of the deferent duct (Bergerson et al., 1994). Presence of some spermatozoa indenting cells apices could explain the spermatophagic role that reported by previous studies including Alexander (1972) in monkey, Flickinger (1975) in rabbit, Riva et al. (1982) in human and Murakami et al. (1986) in dog. The ampulla ductus deferentis showed unclear seasonal variations with PAS and Alcian blue stains. Dense PAS reactivity was more detectable during the winter season than the others; however, Mosallam (1981) stated that the PAS positive substances reached its maximum amount in spring and its minimum in summer. Alcian blue reactivity was moderate in all seasons. Alcian blue /PAS staining also showed granular reactions, indicating presence of acid and netural glycoproteins. Ali et al. (1978) attributed the weak alcainophilia to the presence of a very small amount of sialic acid in the seminal fluids. The ampulla of the camel reacted negatively with Best’s carmine stain, indicating that the ampulla was free from glycogen. These findings were previously confirmed in the ampulla of other mammals such as red deer (Aughey, 1969) and donkey (Abou-Elhamd et al., 2012). However, other studies reported the presence of glycogen in the ampulla ductus deferentis of goats and sheep (Wrobel, 1971). The cells of the lamina epithelialis and the glandular epithelium showed sudanophilic granules, which indicate the presence of lipid droplets. Presence of these droplets could be the origin of the lipid in the seminal plasma (Ali et al., 1978). Chow and Pang (1989) reported in the golden hamster that this gland might produce both fatty and proteinaceous products. Inner circular and outer longitudinal layers of smooth muscle fibers were described previously by Ali et al. (1978), Mosallam (1981) and Goswami et al. (1990) in the camel ampulla. In the present study, the tunica muscularis however was formed of variably arranged smooth muscle bundles in addition to its outer vascular layer, parallet to that recorded by Wrobel and Dellmann (1993) in other domestic animals. |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Conclusion |
||||||||||||||||||||||||||||||||||
|
It is clear that the camel ampulla has a storage function as well as secretory one, and both show variations among different seasons. The different appearances of the apices of lining cells of the ampullary tubules and alveoli demonstrate their various and complex roles in apocrine and merocrine mode of secretions, movement of spermatozoa and secretion in and out of the glandular alveoli, water reabsorption and luminal fluids concentration. The positive reactions of epithelial cells the ampullary glands to Alcian blue, PAS and sudan black stains indicates presence of the acid, neutral glycoprotein and fatty droplet in the seminal fluid, while the negative activity against Best's carmine stain reveals free of the seminal plasma from glycogen. |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Acknowledgments |
||||||||||||||||||||||||||||||||||
|
The authors are grateful to technicians of the Electron Microscopy Unit of Assiut University for their help in processing and imaging of the samples for scanning electron microscopy. |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Conflict of Interests |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
The authors declare no conflict of interest exist. |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
References |
||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Abou-Elhamd, A.S., Salem, A.O., Selim, A.A., 2012. Histological and histochemical studies on the ampulla of the deferent duct of donkey (Equus asinus). Journal of Advanced Veterinary Research 2, 261-270. Abou-Elhamd, A.S., Salem, A.O., Selim, A.A., 2013. Histological and morphometrical studies on the ampulla of the deferent duct of donkey (Equus asinus) in different seasons. Journal of Advanced Veterinary Research 3, 135-141. Alexander, N.J., 1972. Prenatal development of the ductus epididymidis in the rhesus monkey. The effects of fetal castration. Developmental Dynamics 135, 119-134. Ali, H.A., Tingari, M.D., Moniem, K.A., 1978. On the morphology of the accessory male genital glands and histochemistry of the ampulla ductus deferens of the camel (Camelus dromedarius). Journal of Anatomy 125, 227-292. Aughey, E., 1969. Histology and histochemistry of the male accessory glands of red deer, Cervus Elaphus L. Journal of Reproduction and Fertility 18, 399-407. Bancroft, J.D., Layton, C., Suvarna, S.K., 2013. Bancroft's Theory and Practice of Histological Techniques. 7th edn. Churchill Livingstone. Banks, W.J., 1993. Male Reproductive System, Mosby Year Book. 3rd edn. St. Louis, Lo, USA. Bergerson, W., Amselgruber, W., Sinowatz, F., Bergerson, M., 1994. Morphological evidence of sperm maturation in the ampulla ductus deferentis of the bull. Cell and Tissue Research 275, 537-541. Best, F., 1906. Carmine staining of glycogen and nuclei. Zeitschrift fur wissenschaftliche Mikroskopie und mikroskopische Technik 23, 319-322. Chow, P.H., 1988. Scanning electron microscopical study of the seminal vesicle, coagulating gland, ampullary gland and ventral prostate in golden hamster. Acta Anatomica 133, 269-273. Chow, P.H., Pang, S.F., 1989. Ultrastructure of secretory cells of male accessory sex gland of golden hamster (Mesocricetus auratus) and effect of melatonin. Acta Anatomica 134, 327-340. Chughtai, B., Sawas, A., O'Malley, R.L., Naik, R.R., Ali Khan, S., Pentyala, S., 2005. A neglected gland: a review of Cowper's gland. International Journal of Andrology 28, 74-77. Davies Morel, M.C.G., 2003. Equine Reproductive Physiology, Breeding and Stud Management, 2nd edn. CABI Publishing. Wallingford, Oxon, UK. De Duve, C., Wattiaux, R., Baudhuin, P., 1962. Distribution of enzymes between subcellular fractions in animal tissues. Advances in Enzymology and Related Subjects in Biochemistry 24, 291-358. Derar, R.D., Hussein, H.A., Saleh, A.M., 2005. Morphometric and immunohistochemical variations in the camel (Camelus dromedarius) testis in relation to some endocrinological aspects during different seasons of the year. Assiut Veterinary Medical Journal 51, 1-17. Dukes, H.H., 2005. Dukes' Physiology of Domestic Animals, 12th edn. Panima Publishing Corporation, New Delhi. Flickinger, C.J., 1975. Fine Structure of the Rabbit Epididymis and Vas Deferens after Vasectomy. Biology of Reproduction 13, 50–60. Frandson, R.D., Wilke, W.L., Fails, A.D., 2009 Anatomy and Physiology of farm animals. 7th edn. John Wiley and Sons, Inc. Iowa, USA. Goswami, S.K., Sambyal, R.S., Singh, Y., Nagpal, S.K., 1990. Regional histomorphology of the ampulla ductus deferentis of camel. Indian Journal of Animal Sciences 60, 1043-1046. Harris, H.F., 1996. On the rapid conversion of haematoxylin into haematin in staining reactions. Journal of Applied Microscopy and Laboratory Methods 3, 777. In: Theory and Practice of Histological Techniques. Bancroft, J.D., Steven, A. (Eds.),4th edn. Churchill Livingstone, New York, USA. Hinton, B.T., Palladino, M.A., Rudolph, D., Lan, Z.J., Labus, J.C., 1996. The role of the epididymis in the protection of spermatozoa. Current Topics in Developmental Biology 33, 61-102. Khalaf, A.S., Merhish, S.M., 2010. Anatomical study of Accessory Genital Glands in Males Sheep (Ovis aris) and Goats (Caprushircus). The Iraqi Journal of Veterinary Medicine 34, 1-8. Liebich, H.G., 1990. Funktionelle Histologie. Farbatlas und Kurzlehrbuch der mikroskopischen Anatomie der Haussäugetiere. Schattauer. Stuttgart. New York, USA. Lison, L., 1960. Lipides et lipoproteines. In: Histochimie et cytochimie animales. Principes et méthodes. Paris, Gauthier-Villars 2, 449-530. Mai, H.M., 2014. Gross Anatomical Study of the Urogenital System of the Indigenous Nigerian Male Donkey (Equus africanus africanus) in Comparison with the Stallion. Anatomy and Physiology: Current Research 4, Article 1000145. Mahmud, M.A., Josephat, O., Abdullahi, S.S., Aminu, U.M., Abdurrahman, B., Akawu, S.H., Abubakar, D., Shehu, S., 2016. Species Variation on Gross Morphology and Gross Morphometry of Accessory Sex Glands in One-Humped Camel Bull (Camelus dromedarius), Uda Ram and Red Sokoto Buck. World's Veterinary Journal 6, 53-58. McDonald, L.E., 1980. Veterinary Endocrinology and Reproduction, 3rd edn. Lea and Febiger, USA. McManus, J.F., 1946. Histological demonstration of mucin after periodic acid. Nature 158, 202. Mohammed, H.H., Doohi, H.K., 2017. Histomorphology and histochemistry study of ampulla of vas deferens in adult indigenous gazelle (Gazella subgutturosa). Journal of Entomology and Zoology Studies 5, 1452-1455. Mosallam, E. M., 1981. Histological and histochemical studies of the male accessory glands of the adult camel (Camelus dromedarius) in the different seasons of the year. PhD. Thesis, Faculty of Veterinary Medicine, Cairo University, Egypt. Murakami, M., Nishida, T., Shiromoto, M., Inokuchi, T., 1986. Scanning and transmission electron microscopic study of the ampullary region of the dog vas deferens, with special reference to epithelial phagocytosis of spermatozoa and latex beads. Annals of Anatomy 162, 289-296. Nistal, M., Santamaria, L., Paniagua, R., 1992. The ampulla of the ductus deferens in man: morphological and ultrastructural aspects. Journal of Anatomy180, 97-104. Paniagua, R., Regadera, J., Nistal, M., Abaurrea, M.A., 1982. Histological, histochemical and ultrastructural variations along the length of the human vas deferens before and after puberty. Acta Anatomica 111, 190-203. Perera, B.M., 1974. The ampulla of the vas deferens as a source of spermatozoa in the ejaculate of vasectomised ram. Veterinary Records 94, 383-384. Riva, A., Testa-Riva, F., Usai, E., Cossu, U., 1982. The ampulla ductus deferentis in man as viewed by SEM and TEM. Archives of Andrology 8, 157-164. Riva, A., Cossu, M., Usai, E., Scarpa, R., Lantini, M.S., 1989. Fine structure of the accessory glands of the human male genital tract. In: Developments in Ultrastructure of Reproduction. Progress in clinical and biological research. Motta, P.M. (editor). 1st edn. vol. 296.Wiley-Liss Publisher, New York, USA. Saleh, A.M., 2002. Morphological studies on the innervation of the spermatic cord, testis, and epididymis in the camel. PhD. Thesis. Faculty of Veterinary Medicine, Assiut University, Egypt. Setchell, B.P., Maddocks, S., Brooks, D.E., 1994. Anatomy, vasculature, innervation and fluids of the male reproductive tract. In: The Physiology of Reproduction. Knobil, E., Neill, J.D., editors. 2nd edn. Raven Press, New York. Short, R.V., Mann, T., Hay, M.F., 1967. Male reproductive organs of the African elephant, Loxodonta Africana. Journal of Reproduction and Fertility 13, 517-536. Singh, U.B., Bharadwaj, M.B., 1980. Histological studies on the testicular seminal pathway and changes in the epididymis of the camel (Camelus dromedarius). Part IV. Acta Anatomica 108, 481-489. Steedman, H.F., 1950. Alcian blue 8GS: a new stain for mucin. The Quarterly Journal of Microscopical Science 91, 477-479. Thomson, A.A., Marker, P.C., 2006. Branching morphogenesis in the prostate gland and seminal vesicles. Differentiation 74, 382-392. Wrobel, K.H., 1971. The ampulla of the vas deferens in goats. Journal of Veterinary Medicine Series A 18, 250-263. Wrobel, K.H., Dellmann, H.D., 1993. Male reproductive system. In textbook of veterinary histology, 4th edn. Edited by Dellmann, H.D., Lea & Febiger, Philadelphia. |
||||||||||||||||||||||||||||||||||