|
|
Journal of Advanced Veterinary Research Volume 10, Issue 2, 2020, Pages: 81-87 www.advetresearch.com |
|
|
Sesame Oil Ameliorates Gentamicin-induced Cardiotoxicity in Wistar Albino Rats |
|
|
|
Fatma Abo Zakaib A. Ali1*, Nada Abdellah2, Lamiaa O. Hafez3, Ashraf A. El-Ghoneimy4 |
|
|
|
1Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, Sohag University, 82524, Sohag, Egypt. 2Department of Histology, Faculty of Veterinary Medicine, Sohag University, 8252., Sohag, Egypt. 3Department of Pharmacology, Faculty of Veterinary Medicine, Sohag University, 82524, Sohag, Egypt. 4Department of Pharmacology, Faculty of Veterinary Medicine, South Valley University, 83523 Qena, Egypt. |
|
|
|
Received: 20 February 2020; Accepted: 27 March 2020 (*Corresponding author: fatma_ali@vet.sohag.edu.eg) |
|
|
|
Abstract |
|
|
|
Gentamicin (GM) is a widely used aminoglycoside antibiotic with a broad spectrum anti-microbial activity. To the best of the authors’ knowledge, the histopathological studies on the gentamicin myocardial tissue effect are scarce. Therefore, the present study aimed to evaluate the histopathological impact of gentamicin on myocardial tissue besides evaluating the possible cardioprotective role of sesame oil (SEO), and its antioxidant influences against cardiotoxic action of gentamicin in the experimental rats. Forty female Wistar albino rats were divided randomly into four different isolated groups (Ten animals each); Group I was administered normal saline and acts as a control group. Group II was received SEO at a dose of (8 ml/kg daily via gavage) daily for the 10 successive days. Group III was given gentamicin at the dose of (100 mg/kg i.p.) for 10 sequential days. Group IV was given SEO as group II and one-hour latter rats were given gentamicin as in group III. Administration of GM resulted in significant (p≤0.05) structural myocardial alterations in the form of cardiac myofibers disarrangement, hypertrophy; interstitial fibrosis, vascular thrombosis and ischemic infarction. Immunohistochemistry revealed increased iNOS expression levels in GM hearts compared with control groups. Interestingly, concomitant administration of SEO with GM in Group IV revealed a significant reduction in GM‑induced changes, reduced the expression of iNOS and provided a guarding effect to the myocardium. It could be concluded that sesame oil potently may attenuate gentamicin cardiotoxicity by reducing oxidative stress and with possible reduction in nitric oxide level in the hearts of Wistar albino rats. |
|
|
|
Keywords: Cardiotoxicity, Gentamicin, iNOS, Sesame Oil |
|
|
|
Introduction |
|
|
|
The heart is histologically defined in three layers: endocardium, forming of squamous endothelial cells, the myocardium which consists of cardiac muscle fibers that orientated approximately parallel to each other, referring as ‘fibre-orientation’ (Streeter and Hanna, 1973) and a scarce loose connective tissue, the pericardium that has two leaflets (Mescher 2016) Though heart function is intimately connected to myocardial tissue structure, and both change considerably in cardiac disease (Hales et al., 2012). Gentamicin (GM) is one of the aminoglycoside antibiotics frequently used in the treatment of serious infections with aerobic gram-negative and gram-positive bacteria (Testa and Tilley, 1976). However, its frequent use is confined by a risk of toxic effects on some tissues and organs (Prins et al., 1996). Many shreds of evidence over the last decade suppose that the mechanism of gentamicin toxicity due to the deterioration in free radical defense systems including oxidative stress, inflammation, reduced renal blood flow, thereby leading to ischemic, toxic and immune-mediated tissue injury (Serdar Öztürk et al., 1997; Walker et al., 1999). Adding to that, gentamicin was incriminated in increasing nitric oxide (NO) level (Balakumar et al., 2010; Christo et al., 2011), which consider an influential regulator of myocardial contractility, and has been concerned in the incident of heart failure; however, no study exists defining the relation between expression of nitric oxide (NO) synthase (iNOS) in vivo, and cardiac contractility (Heger et al., 2002), it is well determined that nitric oxide (NO) reveals potent cardiovascular actions that include, its vasodilator effect and inhibition of myocardial contractile force development (Kelm et al., 1997), most probably by alteration of mitochondrial respiration (Loke et al., 1999). The most evidenced series side-effects of gentamicin are nephrotoxicity and ototoxicity (Hawkins et al., 1969; Smith et al., 1980; Matz, 1993; Hafez et al., 2019). Moreover, not only the kidney and ear tissue are reported to be affected gentamicin toxicity there are studies showed that the heart tissue may be exposed to oxidative stress. However, the histopathological myocardial changes still not well-defined (Serdar Öztürk et al., 1997), a pathological study on rabbits revealed that long-time administration of gentamycin induces congestion and necrosis of myocardial muscle cells associated with insufficient systemic circulation (Saleh, 2018). Numerous strategies have been designed to protect the tissue from gentamicin toxicity depending on the usage of several antioxidant agents including those extracted from medical plants (Ali, 2003), efficiently reacted with free radicals and discarded the chain reaction earlier before essential molecules are damaged (Lobo et al., 2010). Sesame (Sesamum indicum L) is one of the oldest worldwide cultivated plants. It is extracted from sesame seeds, containing many products with potent antioxidative and anti-inflammatory characteristics as, sesamin, sesamol, episesamin, and sesamolin. Therefore, it improves the hepatic detoxification of chemicals and diminishes the incidence of chemically provoked oxidative stress (Haidari et al., 2016). In view of the above concerns, the present study aimed to define the role of gentamicin-induced histopathological myocardial changes. Also, the protective role of sesame oil (SEO) against gentamicin-induced alterations in histological structure related to myocardial injury. |
|
|
|
Materials and methods |
|
Animals and experimental design Animals Experimental design and all animal handling procedures were approved by the Research Ethical Committee of the Faculty of Veterinary Medicine, South Valley University, Qena, Egypt (Approval No. 20147). Forty female adults Wistar albino rats (weighing about 150-250 g and aged from 3-4 months) were obtained from the animal house, Faculty of Medicine, Assiut University and housed in a separate wire cages under complete isolated conditions in the animal facilities at the Faculty of Veterinary Medicine – Sohag University for two weeks prior to the experiment for acclimation. Animals were kept on a 12 h light, 12 h dark cycle with an ambient temperature of 22-24oC and were provided with a commercial pelleted feed and water ad libitum. Animals were monitored daily for the presence of any illness, and any cause of suffering before the experiment was avoided. Experimental design After adaptation, the forty rats were randomly consigned to four different isolated groups (Ten animals each); Group I was treated with normal saline 10 ml/kg daily via gavage for 10 successive days (Gad et al., 2016) and act as a control group. Group II was received SEO at a dose of 8 ml/kg daily via gavage for 10 successive days (Barakat et al., 2011). Group III was injected with GM at the dose of 100 mg/kg b wt. i.p. daily for 10 consecutive days (Khan et al., 2011). Group IV was given SEO as in group II; one-hour latter, rats were treated with GM as described in group III. Tissue sampling Twenty-four hours after the last treatments, all rats were sacrificed and hearts were quickly cut out from each animal, and fixed directly in 10% neutral buffered formalin to prepare it for histopathological and immunohistochemical assessment. The sampling process performed at the end of the experiment from each group. Histological assessment The formalin-fixed heart specimens were embedded in paraffin wax and sectioned (5μm thickness) and were later stained with Hematoxylin and Eosin (H&E) (Carleton et al., 1980; Bancroft et al., 1996) and Masson's Trichrome stains (specific stain for collagen content) (Sereno et al., 2015). Stained sections were examined by Leitz Dialux Microscope. Photos were taken using Canon digital camera (Canon power shot A95). Quantitative assessment The quantitative evaluation was presented using ImageJ versus 1.48 software (NIH). The myocyte cross-sectional diameter (Fan et al., 2012; Helms et al., 2010) and microscopic scoring of myocardial lesion (Gibson-Corley et al., 2013) were measured using five non-overlapping fields from five different sections from each animal (Abràmoff et al., 2004). Myocyte cross sectional diameter It was measured using H & E‑stained longitudinal sections of LV at ×40 magnification by manually drawing a line around the circumference of cardiomyocytes presented with visible central nuclei. The cross‑sectional means obtained for each group were processed for statistical analysis (Fan et al., 2012; Helms et al., 2010). Ordinal method for validating histopathologic scoring The assigned brief score for each animal based on the tissue histopathological examination (Gibson-Corley et al., 2013). Microscopic myocardial lesions were scored independently by two veterinary pathologists, who were unaware of the treatment or its information in advance, and initial scoring divergences were resolved by mutual re-evaluation. The score was by severity in the examined tissue: 0 = no lesions; 1 = mild (1 to 25% of the tissue section affected); 2 = moderate (25 to 45%); 3 = severe (> 45%) (Gibson-Corley et al., 2013). Immunohistochemical staining Immunohistochemical staining for detection of iNOS was done on 10% formalin-fixed paraffin-embedded myocardial tissue, cut 4–6 μm in thickness and mounted on 3-aminopropyl-trioxysilane (Sigma, St Louis, MO, USA) coated glass slides. Immunostaining for iNOS was performed using the peroxidase–anti peroxidase (PAP) technique. Slides were incubated in 0.3% H2O2 to quench endogenous peroxidase, then boiled for 15 min in citrate buffer, rinsed with PBS and processed for staining. After pre-incubation with normal rabbit serum, slides were incubated overnight at 4oC with mouse monoclonal antibody for iNOS (ab49999, Abcam, Cambridge, UK). After PBS wash, the sections were incubated with rabbit-anti-mouse serum (Dako Corp, Glostrup, Denmark), washed and further incubated with mouse PAP-complex (Sigma,Germany). The chromogen reaction was kept to take place in the dark, using 0.025% 3, 3-diaminobenzidine (Sigma, Germany). Afterward, slides were counterstained with Mayer’s hematoxylin and visualized and photographed underneath a light microscope (Das et al., 2005). Statistical analysis Results data were presented as Mean±S.D. The measurements get from experimental groups were statistically estimated through GraphPad Prism, version 5 (San Diego, California, USA) using one‑way ANOVA with Tukey’s post hoc multiple comparison tests. Statistical significance was determined at P < 0.05. |
|
|
|
Results |
|
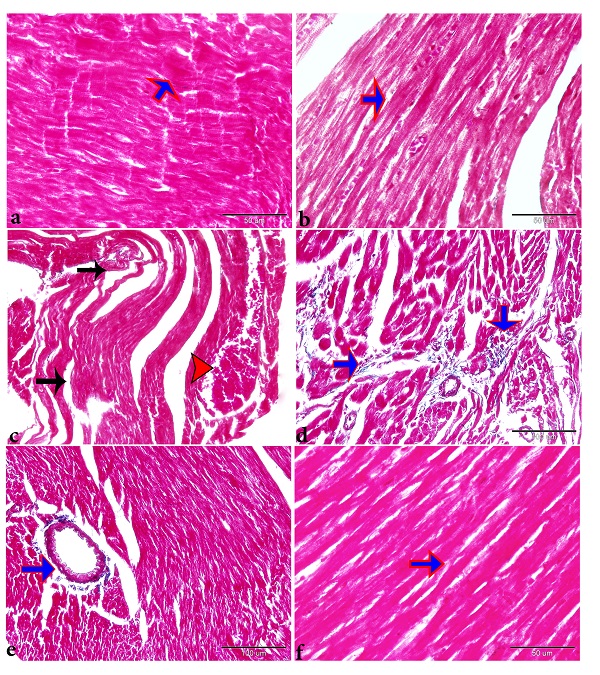
Findings of the histo-pathological and quantitative assessments Myocardial congestion was present in many parts of the hearts. The cardiomyopathic lesions were intense in the myocardial fibers in the left ventricle but were generalized throughout the myocardium of the right ventricle and both atriums. Histological examination of the myocardium revealed that sections that have been taken from control rats appeared with the typical histological architecture of the myocardium with longitudinally striated branching and anastomosing muscle fibers with centrally located oval vesicular nuclei (Figs. 1a, b). However, sections taken from gentamicin‑injected rats showed several pathological findings. These findings include significant (p≤0.05) myocardial fiber hypertrophy compared with the control group; concomitant administration of SEO significantly ameliorated this phenotype compared to the group received GM alone. However, the size of cardiac myocytes in the SEO treated rats remained significantly higher than that of the control group (Fig. 2a). Significant (p≤0.05) disorganization and discontinuation of cardiac muscle fibers (Fig. 1c, d and Fig. 2c), increased intracellular spaces between myocardial fibers, congestion, and thrombosis in blood vessels (BVs), and severe hemorrhage was also recorded in GM treated group compared with the control groups (Figs. 1c, d, Fig. 2b and Fig. 3b, c). Myocardial sections from the gentamicin‑treated group also showed focal areas of infarction with ischemic necrosis that characterized by pale homogeneous acidophilic cytoplasm with either absence of nuclei or presence of deeply stained pyknotic nuclei (Figs. 1c, d). Moreover, mononuclear cellular infiltration in-between cardiac muscle fibers also significantly increased (p≤0.05) in the myocardium of gentamicin‑treated rats (Fig. 1e and Fig. 2d). On the other hand, sections taken from GM rats that were pre-treated with SEO showed a near‑normal structure of the myocardium presented with branching and anastomosing longitudinal muscle fibers with oval vesicular central nuclei (Fig. 1f and Figs. 2a-d).
Fig. 1. Sesame oil ameliorates cardio-toxic effect of gentamicin in adult rat myocardium. Photomicrographs of longitudinal sections of the left ventricular myocardium of (a) control group (b) Sesame oil control group, showing branching and anastomosing cardiac muscle fibers with an acidophilic sarcoplasm and centrally located oval vesicular nuclei (arrowhead). While (c & d) gentamicin treated group showing destruction and discontinuation of cardiac muscle fibers (red arrow), congested blood vessels with extravasated red blood cells in between the muscle fibers (c, black arrow) (d) focal areas of ischemic necrosis (infarction) (white arrowheads) in addition to (e) mononuclear cellular infiltration (white arrows). On the other hand, (f) Pre and co-administration of sesame oil with gentamicin showed a near-normal structure of cardiac muscle fibers (red arrow) with decanting mononuclear cellular infiltration (white arrows). H&E, scale bar = 50 μm, d &e scale bar = 100 μm. Fig. 2. (a) Histomorphometry graph showing quantitative measurements of cardiac myocytes cross‑sectional diameter among the experimental groups. Plots showing quantitative measurements of (b) hemorrhage, (c) necrosis and myofibril arrangement, (d) inflammatory cellular infiltration scoring among the experimental groups. Data are represented as mean ± standard deviation. Significant differences from the control group are marked by asterisks (one‑way ANOVA with Tukey’s post hoc,*P ≤ 0.05. ns; non-significant). Gentamicin injection resulted in increased fibrous tissue content in the myocardium, the increased intracellular space between the cardiac fibers seen in H&E stained sections could possibly be obtained from the loss of the contractile fibers and its replacement by fibrous tissues. To validate this hypothesis, longitudinal sections of the left ventricle were stained with Masson's Trichrome. Gentamicin injection resulted in a significant increase in the area percentage of collagen contents compared to the control group (Figs. 3c&d). On the other hand, co-administration of GM and SEO resulted in a significant decrease in the collagen deposition in‑between the muscle fibers compared to the gentamicin‑treated group (Figs. 3e&f). The results of histopathological analysis thus indicated that the rats' hearts were partially protected by pre-treatment with Sesame oil (8 ml/kg) against gentamicin-induced cardiotoxicity (Figs. 1-3).
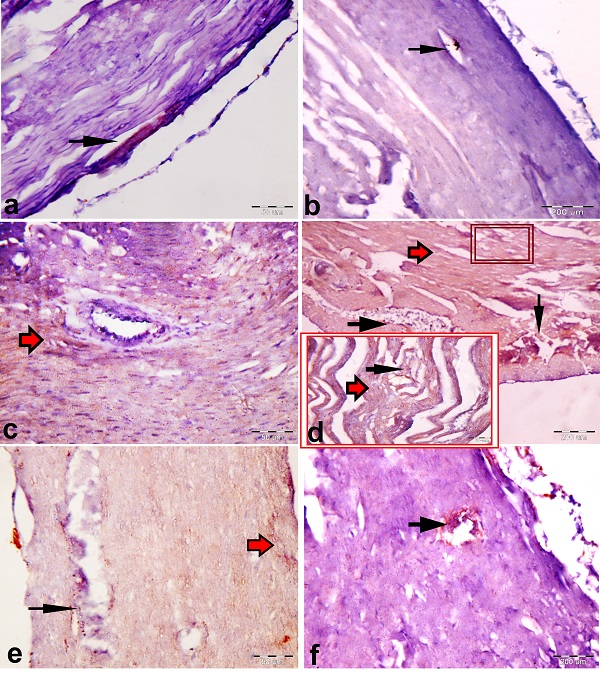
Fig. 3. Demonstrative photomicrographs of the longitudinal sections of left ventricular myocardium presenting (a) few collagen fibers (arrowhead) between the cardiac muscle fibers of the control group (b) Fine collagen fibers (arrowhead) between the cardiac muscle fibers of Sesame oil control group (c &d). An apparent increase in collagen fibers (blue arrows) between the cardiac muscle fibers of the gentamicin‑treated group. Wide intercellular space (black arrow), thrombosis in the blood vessel (red arrow) (e & f). An apparent decrease in the interstitial collagen fibers (blue arrows) with Sesame oil administration. Masson's trichrome stain, scale bar = 50 μm. Immunohistochemistry findings Immunohistochemical localization of iNOS Visualization of the proteins after immunoperoxidase color reaction revealed the increased activity of these proteins in the gentamicin group than rats' hearts from Pre and coadministration of Sesame oil with gentamicin group, all compared with the control group. Immunoreactive iNOS were localized mainly in the cytoplasm of cardiomyocytes and vascular smooth muscle cells and not in fibrotic areas (Figs. 4).
Fig. 4. Immunohistochemical expression of iNOS. Paraffin sections of myocardial tissue obtained from control, sesame oil control, gentamicin, and sesame oil pretreated GM rats respectively, were incubated with antibodies against iNOS. Micrograph showing the immunohistochemical localization of iNOS in the vascular endothelium (black arrow) of the rats employing the peroxidase– anti peroxidase (PAP) method (brown color). (a & b) Endothelial expression of iNOS in coronary vessels in control groups rats. (C) Intense iNOS expression in the endothelium of coronary vessels and cytoplasmic localization in cardiomyocytes in gentamicin treated rats (red arrow). (e&f) Decreased endothelial expression of iNOS in coronary vessels as well as in cardiomyocytes in gentamicin rats pretreated with sesame oil. Bar sizes were indicated under pictures. |
|
|
|
Discussion |
|
To our knowledge, the present study is the first validation of the histopathological impact of gentamicin on myocardial damage in rats besides evaluating the cardio-protective role of sesame oil against gentamicin cardiotoxicity. Gentamicin is often used to prevent Gram-negative infections after cardiac operations (Schersten, 2007, Kozioł et al., 2014). This may be somewhat risky if gentamicin really impairs the free-radical defense system in the heart as it does in the kidney from our previous study (Hafez et al., 2019). The toxic potential of gentamicin on the heart tissue can be seen clearly from our results. Some researchers propose that enzymatic and non-enzymatic free radical scavengers should be given to protect the heart from free radical injury during and after surgical operations (Menasche et al., 1986; Otani et al., 1986). Sesame oil considered as natural antioxidants that may possibly be effective in attempting to lower cardiotoxicity and oxidative stress (Shad et al., 2007). Sesame oil in the present study was found to ameliorate degenerative changes and other cardiac alterations induced by GM administration. This protection was observed at both histologic (H&E) and immunohistochemically stained tissue sections. In the current study, the microscopic observation using H&E pointed out that gentamicin could induce myocardial fiber hypertrophy and widening in intracellular space between the myocardial fibers, which may stem from the loss of the myocardial contractile fibers and its replacement by fibrous tissues. In addition, there were areas of infarction characterized by acute ischemic coagulation necrosis of myocardial tissue due to obstruction of its arterial blood supply by thrombus, thus in addition to, mononuclear cellular infiltration in between the muscle fibers. The ischemic action of gentamicin on the heart was approved in previous studies (Serdar Öztürk et al., 1997) Rats pretreated with SEO provided marked histological protection against GM-induced myocardial damage. The obtained results confirmed that SEO was efficiently protect against gentamicin cardio-toxicity. This finding is confirmed by Shad et al. (2007), who confirmed the myocardial- protective role of SEO is attributed to its antioxidant properties. It is well known that various cultured cell types including cardiac myocytes and myocardial microvascular endothelial cells are able to produce NO (Luss et al., 1994). The in vivo anatomical localization of iNOS in the heart has been a matter of argument (De Belder et al., 1993). iNOS expression was approved linked to the condition of heart failure intrinsically rather than connected to heart failure etiology (Vejlstrup et al., 1998). In the present study, gentamicin-treated group showed high nitrite/nitrate tissue expression. The inducible nitric oxide (NO) synthase (iNOS or NOS2) creates a prolonged release of high amounts of NO, which may be cytotoxic or inhibit myocyte contractility. It has been proposed that this mechanism particularly contributes to heart failure caused by dilated cardiomyopathy (Vejlstrup et al., 1998). This study demonstrated that sesame oil protected against gentamicin cardiotoxicity in rats. That may back to the ability of sesame oil to reduce oxidative stress, generation of reactive oxygen species, nitric oxide levels, and iNOS expression levels in rats (Hafez et al., 2019). Prior studies revealed that sesame oil is an effective scavenger of hydroxyl radicals (Hsu et al., 2004; Hsu and Liu, 2004). Sesame oil inhibiting nitric oxide generation may be associated with sesame oil’s protection against gentamicin. During oxidative stress, most of the hydroxyl radical is generated via the reaction of superoxide anion and nitric oxide (Beckman et al., 1990; Gross and Wolin, 1995), whereas nitric oxide is mostly generated by iNOS (Yasmin et al., 1997). Sesame oil reduced iNOS generation and expression (Hsu et al., 2008). This result is in agreement with the findings of previous studies (Hsu et al., 2008). Therefore, the present study suggests that sesame oil acts as an inhibitor of iNOS expression and plays a crucial role in cardiac protection against gentamicin toxicity. |
|
|
|
Conclusion |
|
Sesame Oil offers protection to the myocardium through its antioxidant effects against iNOS expression levels. The authors recommend further biochemical and physio-pathological studies on the gentamicin cardio-toxic effect. |
|
|
|
Conflict of Interests |
|
The authors declare that there is no conflict of interest exists. |
|
|
|
References |
|
|
|
Abràmoff, M.D., Magalhães, P.J., Ram, S.J., 2004. Image processing with ImageJ. Biophotonics international 11, 36-42. Ali S.S., Muzaffar, S., Ahmad, A., Ali, A., Hassan, S., 2003. Renalcortical necrosis: a case series of nine patients & review of literature. . J. Ayub. Med. Coll. Abbottabad. 15, 41-44. Balakumar, P., Rohilla, A., Thangathirupathi, A., 2010. Gentamicin-induced nephrotoxicity: do we have a promising therapeutic approach to blunt it? Pharmacological Research 62, 179-186. Gibson-Corley, K.N., Olivier, A.K., Meyerholz, D.K., 2013. Principles for valid histopathologic scoring in research. Veterinary Pathology 50, 1007-1015. Mescher, A., 2016. Junqueira's Basic Histology Text & Atlas (14th ed.). |
|
|